Spiroplasma endosymbiont reduction of host lipid synthesis and Stomoxyn-like peptide contribute to trypanosome resistance in the tsetse fly Glossina fuscipes
- PMID: 39888974
- PMCID: PMC11819587
- DOI: 10.1371/journal.ppat.1012692
Spiroplasma endosymbiont reduction of host lipid synthesis and Stomoxyn-like peptide contribute to trypanosome resistance in the tsetse fly Glossina fuscipes
Abstract
Tsetse flies (Glossina spp.) vector African trypanosomes that cause devastating diseases in humans and domestic animals. Within the Glossina genus, species in the Palpalis subgroup exhibit greater resistance to trypanosome infections compared to those in the Morsitans subgroup. Varying microbiota composition and species-specific genetic traits can significantly influence the efficiency of parasite transmission. Notably, infections with the endosymbiotic bacterium Spiroplasma have been documented in several Palpalis subgroup species, including Glossina fuscipes fuscipes (Gff). While Spiroplasma infections in Gff are known to hinder trypanosome transmission, the underlying mechanisms remain unknown. To investigate Spiroplasma-mediated factors affecting Gff vector competence, we conducted high-throughput RNA sequencing of the gut tissue along with functional assays. Our findings reveal elevated oxidative stress in the gut environment in the presence of Spiroplasma, evidenced by increased expression of nitric oxide synthase, which catalyzes the production of trypanocidal nitric oxide. Additionally, we observed impaired lipid biosynthesis leading to a reduction of this important class of nutrients essential for parasite and host physiologies. In contrast, trypanosome infections in Gff's midgut significantly upregulated various immunity-related genes, including a small peptide, Stomoxyn-like, homologous to Stomoxyn first discovered in the stable fly, Stomoxys calcitrans. We observed that the Stomoxyn-like locus is exclusive to the genomes of Palpalis subgroup tsetse species. GffStomoxyn is constitutively expressed in the cardia (proventriculus) and synthetic GffStomoxyn exhibits potent activity against Escherichia coli and bloodstream form of Trypanosoma brucei parasites, while showing no effect against insect stage procyclic forms or tsetse's commensal endosymbiont Sodalis in vitro. Reducing GffStomoxyn levels significantly increased trypanosome infection prevalence, indicating its potential trypanocidal role in vivo. Collectively, our results suggest that the enhanced resistance to trypanosomes observed in Spiroplasma-infected Gff may be due to the reduced lipid availability necessary for parasite metabolic maintenance. Furthermore, GffStomoxyn could play a crucial role in the initial immune response(s) against mammalian parasites early in the infection process in the gut and prevent gut colonization. We discuss the molecular characteristics of GffStomoxyn, its spatial and temporal expression regulation and its microbicidal activity against Trypanosome parasites. Our findings reinforce the nutritional influences of microbiota on host physiology and host-pathogen dynamics.
Copyright: © 2025 Awuoche et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
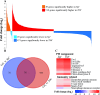


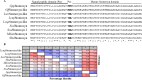
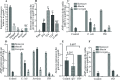
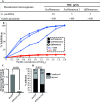
Update of
-
Spiroplasma endosymbiont reduction of host lipid synthesis and Stomoxyn-like peptide contribute to trypanosome resistance in the tsetse fly Glossina fuscipes.bioRxiv [Preprint]. 2024 Oct 24:2024.10.24.620045. doi: 10.1101/2024.10.24.620045. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Jan 31;21(1):e1012692. doi: 10.1371/journal.ppat.1012692. PMID: 39484388 Free PMC article. Updated. Preprint.
Similar articles
-
Spiroplasma endosymbiont reduction of host lipid synthesis and Stomoxyn-like peptide contribute to trypanosome resistance in the tsetse fly Glossina fuscipes.bioRxiv [Preprint]. 2024 Oct 24:2024.10.24.620045. doi: 10.1101/2024.10.24.620045. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Jan 31;21(1):e1012692. doi: 10.1371/journal.ppat.1012692. PMID: 39484388 Free PMC article. Updated. Preprint.
-
Spatio-temporal distribution of Spiroplasma infections in the tsetse fly (Glossina fuscipes fuscipes) in northern Uganda.PLoS Negl Trop Dis. 2019 Aug 1;13(8):e0007340. doi: 10.1371/journal.pntd.0007340. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31369548 Free PMC article.
-
Infection with endosymbiotic Spiroplasma disrupts tsetse (Glossina fuscipes fuscipes) metabolic and reproductive homeostasis.PLoS Pathog. 2021 Sep 16;17(9):e1009539. doi: 10.1371/journal.ppat.1009539. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34529715 Free PMC article.
-
Innate immunity in the tsetse fly (Glossina), vector of African trypanosomes.Dev Comp Immunol. 2019 Sep;98:181-188. doi: 10.1016/j.dci.2019.05.003. Epub 2019 May 7. Dev Comp Immunol. 2019. PMID: 31075296 Review.
-
Bacterial Symbionts of Tsetse Flies: Relationships and Functional Interactions Between Tsetse Flies and Their Symbionts.Results Probl Cell Differ. 2020;69:497-536. doi: 10.1007/978-3-030-51849-3_19. Results Probl Cell Differ. 2020. PMID: 33263885 Review.
References
-
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). 2019. [cited 2023 November 5, 2019]. Available from: www.who.int/news-room/fact-sheets/detail/ trypanosomiasis- human- african- (sleeping- sickness).
-
- Holt HR, Selby R, Mumba C, Napier GB, Guitian J. Assessment of animal African trypanosomiasis (AAT) vulnerability in cattle-owning communities of sub-Saharan Africa. Parasites & vectors. 2016;9:53. Epub 2016/01/31. doi: 10.1186/s13071-016-1336-5 ; PubMed Central PMCID: PMC4733274. - DOI - PMC - PubMed
-
- Maudlin I, Holmes PH, Miles MA. The Trypanosomiasis. CAB International. Wallingford, UK.: CABI Publishing.; 2004.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

