Monoaminergic neurotransmitters are bimodal effectors of tau aggregation
- PMID: 39888993
- PMCID: PMC11784839
- DOI: 10.1126/sciadv.adr8055
Monoaminergic neurotransmitters are bimodal effectors of tau aggregation
Abstract
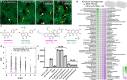
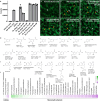
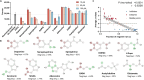
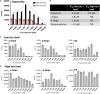
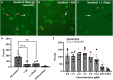
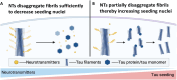
Neurotransmitters (NTs) mediate trans-synaptic signaling, and disturbances in their levels are linked to aging and brain disorders. Here, we ascribe an additional function for NTs in mediating intracellular protein aggregation by interaction with cytosolic protein fibrils. Cell-based seeding experiments revealed monoaminergic NTs as inhibitors of tau. Seeding is a disease-relevant mechanism involving catalysis by fibrils, leading to the aggregation of proteins in Alzheimer's disease and other neurodegenerative diseases. Chemotyping small molecules with varied backbone structures revealed determinants of aggregation inhibitors and catalysts. Among those identified were monoaminergic NTs. Dose titrations revealed bimodal effects indicative of fibril disaggregation, with aggregation catalysis occurring at low ratios of NTs and inhibited seeding ensuing at higher concentrations. Bimodal effects by NTs extend from in vitro systems to dopaminergic neurons, suggesting that pharmacotherapies that modify intracellular NT levels could shape the neuronal protein aggregation environment.
Figures

References
-
- van Dyck C. H., Swanson C. J., Aisen P., Bateman R. J., Chen C., Gee M., Kanekiyo M., Li D., Reyderman L., Cohen S., Froelich L., Katayama S., Sabbagh M., Vellas B., Watson D., Dhadda S., Irizarry M., Kramer L. D., Iwatsubo T., Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023). - PubMed
-
- Clavaguera F., Akatsu H., Fraser G., Crowther R. A., Frank S., Hench J., Probst A., Winkler D. T., Reichwald J., Staufenbiel M., Ghetti B., Goedert M., Tolnay M., Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. U.S.A. 110, 9535–9540 (2013). - PMC - PubMed
-
- Sanders D. W., Kaufman S. K., DeVos S. L., Sharma A. M., Mirbaha H., Li A., Barker S. J., Foley A. C., Thorpe J. R., Serpell L. C., Miller T. M., Grinberg L. T., Seeley W. W., Diamond M. I., Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). - PMC - PubMed
-
- Seidler P. M., Murray K. A., Boyer D. R., Ge P., Sawaya M. R., Hu C. J., Cheng X., Abskharon R., Pan H., DeTure M. A., Williams C. K., Dickson D. W., Vinters H. V., Eisenberg D. S., Structure-based discovery of small molecules that disaggregate Alzheimer’s disease tissue derived tau fibrils in vitro. Nat. Commun. 13, 5451 (2022). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

