Prognostic Models From Transcriptomic Signatures of the Tumor Microenvironment and Cell Cycle in Stage III Colon Cancer From PETACC-8 and IDEA-France Trials
- PMID: 39889251
- PMCID: PMC12084023
- DOI: 10.1200/JCO.23.02262
Prognostic Models From Transcriptomic Signatures of the Tumor Microenvironment and Cell Cycle in Stage III Colon Cancer From PETACC-8 and IDEA-France Trials
Abstract
Purpose: The objective of this work was to establish prognostic models in stage III colon cancer (CC) on the basis of transcriptomic signatures of the tumor microenvironment (TME) and cell cycle from the PETACC-8 (training set) and IDEA-France (validation set) trials.
Patients and methods: 3'RNA sequencing was performed in 1,733 patients from the PETACC-8 trial and 1,248 patients from the IDEA-France trial. Four transcriptomic signatures were analyzed: T-cell and macrophage M2 signatures, the expression of CXCL13, and a score on the basis of the Oncotype DX CC Recurrence Score using the same formula from the stromal score and the cell cycle score. The Immune Proliferative Stromal (IPS) score was defined as the number of dichotomized signatures that fall under the category of a dismal prognosis (from 0 to 4). Time to recurrence (TTR) was defined as the time from the date of random assignment to local and/or metastatic relapse and/or death because of CC, whichever occurs first.
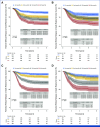
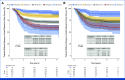
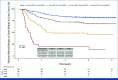
Results: High Oncotype-like and M2 scores and low CXCL13 expression and T-cell score were associated with a shorter TTR. A multivariable model including these signatures and all known prognostic factors applied to the IDEA-France cohort by obtaining a value of this model for each patient showed TTR significantly different depending on the quartile of this value and a 3-year rate of patients without recurrence ranging from 56% for the lowest quartile to 89% for the highest quartile (P < .0001). The IPS score was significantly associated with TTR in multivariable analysis.
Conclusion: Using transcriptomic data of patients with stage III CC from two large-scale adjuvant trials, a prognostic model on the basis of signatures of the TME and the cell cycle provides important information in addition to known prognostic factors for patient stratification on risk of recurrence.
Trial registration: ClinicalTrials.gov NCT00958737.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Grothey A, Venook AP: Optimizing adjuvant therapy for localized colon cancer and treatment selection in advanced colorectal cancer. J Natl Compr Cancer Netw 16:611-615, 2018 - PubMed
-
- Argilés G, Tabernero J, Labianca R, et al. : Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1291-1305, 2020 - PubMed
-
- Sinicrope FA, Huebner LJ, Laurent-Puig P, et al. : Relative contribution of clinical and molecular features to outcome within low and high risk T and N groups in stage III colon cancer (CC). J Clin Oncol 37, 2019. (suppl 15; abstr 3520)
-
- Pagès F, Mlecnik B, Marliot F, et al. : International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 391:2128-2139, 2018 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

