Estimating COVID-19 associated hospitalizations, ICU admissions, and in-hospital deaths averted in the United States by 2023-2024 COVID-19 vaccination: A conditional probability, causal inference, and multiplier-based approach
- PMID: 39889531
- PMCID: PMC12168029
- DOI: 10.1016/j.vaccine.2025.126808
Estimating COVID-19 associated hospitalizations, ICU admissions, and in-hospital deaths averted in the United States by 2023-2024 COVID-19 vaccination: A conditional probability, causal inference, and multiplier-based approach
Abstract
COVID-19-associated hospitalizations, ICU admissions, and in-hospital deaths averted from 2023 to 2024 COVID-19 vaccination from the weeks of October 1, 2023, through April 21, 2024, were estimated via a novel multiplier model that utilized causal inference, conditional probabilities of hospitalization, and correlations between data elements in Monte Carlo simulations. Median COVID-19-associated hospitalizations averted were 68,315 (95 % uncertainty interval [UI] 42,831-97,984), ICU admissions averted were 13,108 (95 % UI 4459-25,042), and in-hospital deaths averted were 5301 (95 % UI 101-14,230). Averted COVID-19-associated burden was highest in adults aged 65 years and older (hospitalizations averted 57,665, 95 % UI 35,442-84,006; ICU admissions averted 10,878, 95 % UI 3104-21,591; in-hospital deaths averted 4779, 95 % UI 0-13,132). Expanding the analytic period to comprise the weeks of September 24, 2023, through August 11, 2024, resulted in 107,197 COVID-19-associated hospitalizations averted (95 % UI 80,692-137,643), 18,292 COVID-19-associated ICU admissions averted (95 % UI 10,062-28,436), and 6749 COVID-19-associated in-hospital deaths averted (95 % UI 2077-13,557). Older adults had the highest COVID-19-associated averted burden and potential to reduce burden further through increased vaccine coverage. 2023-2024 COVID-19 vaccinations reduced the burden of COVID-19-associated severe disease.
Keywords: COVID-19; disease burden; epidemiological models; vaccines.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
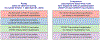

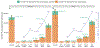
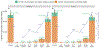
References
-
- Di Fusco M, Marczell K, Deger KA, et al. Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. Journal of Medical Economics 2022;25(1):605–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

