Neoadjuvant immunotherapy with or without chemotherapy in locally advanced oral squamous cell carcinoma: Randomized, two-arm, phase 2 trial
- PMID: 39889711
- PMCID: PMC11866509
- DOI: 10.1016/j.xcrm.2025.101930
Neoadjuvant immunotherapy with or without chemotherapy in locally advanced oral squamous cell carcinoma: Randomized, two-arm, phase 2 trial
Abstract
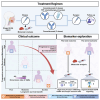
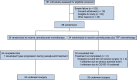
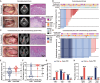
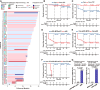
Patients with locally advanced oral squamous cell carcinoma (OSCC) have poor outcomes with standard care. Neoadjuvant therapy is shown to be effective for these patients. In the randomized, two-arm, phase 2, non-comparative trial, we investigate the efficacy and safety of the neoadjuvant programmed cell death 1 (PD-1) inhibitor camrelizumab with or without docetaxel-cisplatin-5-fluorouracil (TPF) chemotherapy in patients with resectable locally advanced OSCC. Patients with stage III-IVA OSCC receive neoadjuvant therapy with three cycles of camrelizumab (arm Cam) with or without two cycles of TPF chemotherapy (arm Cam+TPF), followed by surgery and adjuvant therapy. Major pathological response (MPR) is achieved in both arm Cam (5/34, 14.7%) and arm Cam+TPF (26/34, 76.4%). With a median follow-up of 32 months, the 2-year event-free survival (EFS) rate of arm Cam and Cam+TPF is 52.9% and 91.2%, respectively. This work demonstrates feasibility and safety for immunochemotherapy in the neoadjuvant setting for OSCC. This study was registered at ClinicalTrials.gov (NCT04649476).
Keywords: PD-1 inhibitor; chemotherapy; neoadjuvant therapy; oral squamous cell carcinoma; pathological response.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Gatta G., Botta L., Sánchez M.J., Anderson L.A., Pierannunzio D., Licitra L., EUROCARE Working Group: Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer. 2015;51:2130–2143. doi: 10.1016/j.ejca.2015.07.043. - DOI - PubMed
-
- Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefèbvre J.-L., Greiner R.H., Giralt J., Maingon P., Rolland F., Bolla M., et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. - DOI - PubMed
-
- Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

