Disrupted metabolic flux balance between pyruvate dehydrogenase and pyruvate carboxylase in human fatty liver
- PMID: 39890055
- PMCID: PMC11955189
- DOI: 10.1016/j.metabol.2025.156151
Disrupted metabolic flux balance between pyruvate dehydrogenase and pyruvate carboxylase in human fatty liver
Abstract
Hepatic metabolism involving pyruvate carboxylase (PC) and pyruvate dehydrogenase (PDH) may be abnormal in fatty livers. In this study, [13C]bicarbonate production from [1-13C1]pyruvate in the liver and glycerol glyceroneogenesis were examined in relation to hepatic fat content using hyperpolarized [1-13C1]pyruvate and oral [U-13C3]glycerol. After an overnight fast, 15 subjects with a range of hepatic fat content received hyperpolarized [1-13C1]pyruvate intravenously to assess its conversion to [1-13C1]lactate and [13C]bicarbonate in the liver. They also received oral [U-13C3]glycerol, followed by venous blood sampling to examine glucose and the glycerol backbone of the triglycerides released primarily from the liver. From hyperpolarized [1-13C1]pyruvate, participants with high intrahepatic fat fraction produced higher [1-13C1]lactate and lower [13C]bicarbonate than those with low liver fat. The fraction of plasma triglycerides derived from oral [U-13C3]glycerol via the TCA cycle was similar between groups. The fraction of plasma [5,6-13C2]glucose, which reflects PC flux, decreased in subjects with fatty liver. In contrast, the fraction of [4,5-13C2]glucose + [6-13C1]glucose, which can be produced via either PC or PDH, was comparable between groups. The study results suggest a shift in pyruvate metabolism in fatty liver, with a decreased metabolic flux ratio of PC/PDH. The methodology in this study provides insights into fatty liver metabolism of human subjects inaccessible previously and is applicable to advanced liver diseases such as cirrhosis and hepatomas.
Keywords: Fatty liver; Glyceroneogenesis; Hyperpolarized; Pyruvate carboxylase; Pyruvate dehydrogenase.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Authors declare that they have no competing interests.
Figures

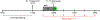
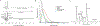


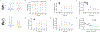

References
-
- Jitrapakdee S, Gong Q, MacDonald MJ, Wallace JC. Regulation of rat pyruvate carboxylase gene expression by alternate promoters during development, in genetically obese rats and in insulin-secreting cells. Multiple transcripts with 5'-end heterogeneity modulate translation. J Biol Chem. Dec 18 1998;273(51):34422–8. doi: 10.1074/jbc.273.51.34422 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

