Durability of immunogenicity at 5 years after a single dose of human papillomavirus vaccine compared with two doses in Tanzanian girls aged 9-14 years: results of the long-term extension of the DoRIS randomised trial
- PMID: 39890232
- PMCID: PMC11783036
- DOI: 10.1016/S2214-109X(24)00477-7
Durability of immunogenicity at 5 years after a single dose of human papillomavirus vaccine compared with two doses in Tanzanian girls aged 9-14 years: results of the long-term extension of the DoRIS randomised trial
Abstract
Background: WHO has recommended that one dose of human papillomavirus (HPV) vaccine can be given to individuals aged 9-20 years to prevent HPV infection. Estimating durability of immune responses after a single dose in the target age for vaccination is important. We report immunogenicity results in Tanzanian girls up to 5 years after receiving a dose.
Methods: In this open-label, randomised controlled trial (the Dose Reduction Immunobridging and Safety Study of Two HPV Vaccines in Tanzanian Girls [DoRIS] trial), 930 Tanzanian schoolgirls aged 9-14 years were enrolled and randomly allocated to receive one, two, or three doses of either the two-valent vaccine (Cervarix; GSK, Wavre, Belgium) or nine-valent vaccine (Gardasil-9; Merck Sharp & Dohme, Haarlem, Netherlands). Seropositivity specific to HPV16 or HPV18, antibody geometric mean concentrations (GMCs), and antibody avidity were measured annually up to month 36. Participants in the one-dose and two-dose groups were followed annually in a long-term extension of the DoRIS trial to month 60; the primary outcome was seropositivity specific to HPV16 or HPV18 comparing one dose with two doses.
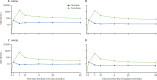
Findings: Single-dose seropositivity for HPV16 IgG antibodies at month 60 with either vaccine was more than 99% and non-inferior to two doses. 98% of girls in the one-dose two-valent vaccine group and 93% in the one-dose nine-valent group were seropositive for HPV18 at month 60; however, the non-inferiority criteria for HPV18 seropositivity comparing one dose with two doses were not met. Although HPV16 and HPV18 antibody GMCs after one dose were lower than those observed after two doses, antibody GMCs in the one-dose groups remained stable from month 12 to month 60. There was no evidence of a difference between the one-dose and two-dose groups in HPV16 or HPV18 antibody avidity at month 36 for either vaccine.
Interpretation: A single dose of HPV vaccine in girls aged 9-14 years continues to provide stable immune responses 5 years after vaccination, although ongoing surveillance for potential waning immunity after a single dose is needed. Participants are being followed up to 9 years after vaccination.
Funding: UK Department of Health and Social Care, UK Foreign, Commonwealth & Development Office, Global Challenges Research Fund, UK Medical Research Council, and the Wellcome Trust through the Joint Global Health Trials Scheme; Bill & Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DW-J reports grants from the Bill & Melinda Gates Foundation and GSK Biologicals. KB reports grants from the Bill & Melinda Gates Foundation and Merck. All other authors declare no competing interests.
Figures
References
-
- WHO Global Strategy to accelerate the elimination of cervical cancer as a public health problem. 2020. https://www.who.int/publications/i/item/9789240014107
-
- WHO Human papillomavirus (HPV) vaccination coverage. https://immunizationdata.who.int/pages/coverage/hpv.html
-
- Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36:4761–4767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

