Artificial intelligence-guided detection of under-recognised cardiomyopathies on point-of-care cardiac ultrasonography: a multicentre study
- PMID: 39890242
- PMCID: PMC12084816
- DOI: 10.1016/S2589-7500(24)00249-8
Artificial intelligence-guided detection of under-recognised cardiomyopathies on point-of-care cardiac ultrasonography: a multicentre study
Abstract
Background: Point-of-care ultrasonography (POCUS) enables cardiac imaging at the bedside and in communities but is limited by abbreviated protocols and variation in quality. We aimed to develop and test artificial intelligence (AI) models to screen for under-diagnosed cardiomyopathies from cardiac POCUS.
Methods: In a development set of 290 245 transthoracic echocardiographic videos across the Yale-New Haven Health System (YNHHS), we used augmentation approaches, and a customised loss function weighted for view quality to derive a POCUS-adapted, multi-label, video-based convolutional neural network that discriminates hypertrophic cardiomyopathy and transthyretin amyloid cardiomyopathy from controls without known disease. We evaluated the model across independent, internal, and external, retrospective cohorts of individuals undergoing cardiac POCUS across YNHHS and the Mount Sinai Health System (MSHS) emergency departments (between 2012 and 2024) to prioritise key views and validate the diagnostic and prognostic performance of single-view screening protocols.
Findings: Between Nov 1, 2023, and March 28, 2024, we identified 33 127 patients (mean age 58·9 [SD 20·5] years, 17 276 [52·2%] were female, 14 923 [45·0%] were male, and for 928 [2·8%] sex was recorded as unknown) at YNHHS and 5624 patients (mean age 56·0 [20·5] years, 1953 [34·7%] were female, 2470 [43·9%] were male, and for 1201 [21·4%] sex was recorded as unknown) at MSHS with 78 054 and 13 796 eligible cardiac POCUS videos, respectively. AI deployed to single-view POCUS videos successfully discriminated hypertrophic cardiomyopathy (eg, area under the receiver operating characteristic curve 0·903 [95% CI 0·795-0·981] in YNHHS; 0·890 [0·839-0·938] in MSHS for apical-4-chamber acquisitions) and transthyretin amyloid cardiomyopathy (0·907 [0·874-0·932] in YNHHS; 0·972 [0·959-0·983] in MSHS for parasternal acquisitions). In YNHHS, 40 (58%) of 69 hypertrophic cardiomyopathy cases and 22 (46%) of 48 transthyretin amyloid cardiomyopathy cases would have had a positive screen by AI-POCUS at a median of 2·1 (IQR 0·9-4·5) years and 1·9 (0·6-3·5) years before diagnosis. Moreover, among 25 261 participants without known cardiomyopathy followed up over a median of 2·8 (1·2-6·4) years, AI-POCUS probabilities in the highest (vs lowest) quintile for hypertrophic cardiomyopathy and transthyretin amyloid cardiomyopathy conferred a 17% (adjusted hazard ratio 1·17, 95% CI 1·06-1·29; p=0·0022) and 32% (1·39, 1·19-1·46; p<0·0001) higher adjusted mortality risk, respectively.
Interpretation: We developed and validated an AI framework that enables scalable, opportunistic screening of under-recognised cardiomyopathies through simple POCUS acquisitions.
Funding: National Heart, Lung, and Blood Institute, Doris Duke Charitable Foundation, and BridgeBio.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC-BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RK is an Associate Editor of JAMA and receives research support, through Yale, from the Blavatnik Foundation, Bristol-Myers Squibb, Novo Nordisk, and BridgeBio. He is a coinventor of pending patent applications (WO2023230345A1, US20220336048A1, 63/346,610, 63/484,426, 63/508,315, 63/580,137, 63/606,203, 63/619,241, and 63/562,335), and a co-founder of Ensight-AI and Evidence2Health. EKO is a co-founder of Evidence2Health, a co-inventor of patent applications (18/813,882, 17/720,068, 63/619,241, 63/177,117, 63/580,137, 63/606,203, 63/562,335, and US11948230B2), has been a consultant for Caristo Diagnostics and Ensight-AI, and has received royalty fees from technology licensed through the University of Oxford (Oxford, UK), outside this work. HMK has received grants and contracts from the American Heart Association, the National Institutes of Health, the Centers for Medicare & Medicaid Services, US Centers for Disease Control and Prevention, Janssen, Kenvue, Novartis, and Pfizer, all outside this work and through Yale University or Yale–New Haven Hospital (New Haven, CT, USA). He has received consulting fees from the Massachusetts Medical Society as Co-Editor for the Journal Watch–Cardiology, as Section Editor for UpToDate, has received stock options for advisory roles from Element Science and Identifeye, and is a co-founder of Hugo Health, Refactor Health, and Ensight-AI. All other authors declare no competing interests.
Figures


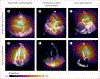
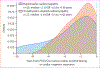
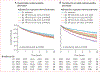
Update of
-
Artificial intelligence-guided detection of under-recognized cardiomyopathies on point-of-care cardiac ultrasound: a multi-center study.medRxiv [Preprint]. 2024 Jun 29:2024.03.10.24304044. doi: 10.1101/2024.03.10.24304044. medRxiv. 2024. Update in: Lancet Digit Health. 2025 Feb;7(2):e113-e123. doi: 10.1016/S2589-7500(24)00249-8. PMID: 38559021 Free PMC article. Updated. Preprint.
Comment in
-
Exploring electronic health records to study rare diseases.Lancet Digit Health. 2025 Feb;7(2):e103. doi: 10.1016/j.landig.2025.01.008. Lancet Digit Health. 2025. PMID: 39890237 No abstract available.
References
-
- Spencer KT, Flachskampf FA. Focused cardiac ultrasonography. JACC Cardiovasc Imaging 2019; 12: 1243–53. - PubMed
-
- Dessie AS, Calhoun AW, Kanjanauptom P, et al. Development and validation of a point-of-care-ultrasound image quality assessment tool: the POCUS IQ scale. J Ultrasound Med 2023; 42: 135–45. - PubMed
-
- Ommen SR, Ho CY, Asif IM, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024; 149: e1239–311. - PubMed
-
- Kittleson MM, Ruberg FL, Ambardekar AV, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023; 81: 1076–126. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

