Glucomannan engineering highlights roles of galactosyl modification in fine-tuning cellulose-glucomannan interaction in Arabidopsis cell walls
- PMID: 39890794
- PMCID: PMC11785759
- DOI: 10.1038/s41467-025-56626-y
Glucomannan engineering highlights roles of galactosyl modification in fine-tuning cellulose-glucomannan interaction in Arabidopsis cell walls
Abstract
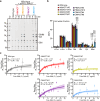
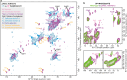
Widely found in most plant lineages, β-mannans are structurally diverse polysaccharides that can bind to cellulose fibrils to form the complex polysaccharide architecture of the cell wall. How changes in polysaccharide structure influence its cell wall solubility or promote appropriate interaction with cellulose fibrils is poorly understood. Glucomannan backbones acquire variable patterns of galactosyl substitutions, depending on plant developmental stage and species. Here, we show that fine-tuning of galactosyl modification on glucomannans is achieved by the differing acceptor recognition of mannan α-galactosyltransferases (MAGTs). Biochemical analysis and 13C solid-state nuclear magnetic resonance spectroscopy of Arabidopsis with cell wall glucomannan engineered by MAGTs reveal that the degree of galactosylation strongly affects the interaction with cellulose. The findings indicate that plants tailor galactosyl modification on glucomannans for constructing an appropriate cell wall architecture, paving the way to convert properties of lignocellulosic biomass for better use.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

