Systematic study on the evaluation method of surface antibacterial activity based on the fluorescent observation of bacterial growth
- PMID: 39890805
- PMCID: PMC11785733
- DOI: 10.1038/s41598-024-81945-3
Systematic study on the evaluation method of surface antibacterial activity based on the fluorescent observation of bacterial growth
Abstract
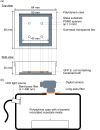
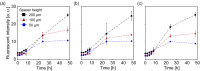
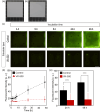
Antibacterial and antiviral coating materials have attracted increasing attention for the prevention of infections caused by frequently touched surfaces in communities and hospitals. The standard assessment procedure for antibacterial surfaces involves bacterial culture on a film-covered substrate followed by transfer onto agar for colony counting (ISO22196:2011). However, this assessment lacks temporal and spatial information regarding bacterial growth, resulting in an incomplete and inaccurate evaluation of the antibacterial activity of the surface. In this study, we develop a novel evaluation procedure for antibacterial substrates that enables in situ visualization of bacterial growth on a surface with centimeter-scale spatial information using fluorescent protein-expressing bacterial cells and an image acquisition setup. The effects of equipment parameters on bacterial growth are systematically investigated to establish the standard evaluation conditions. Based on the optimized parameters, a quantitative evaluation of the antibacterial activity of the coating material is successfully demonstrated. The proposed method is expected to be useful in investigating the spatial and temporal distribution of bacterial growth on substrates.
Keywords: Antibacterial activity; Bacterial growth; Fluorescent observation; in situ visualization.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: This study was a collaborative research project between S. O. and Nippon Paint Co. Ltd, and was funded by Nippon Paint Co. Ltd. D. H., R. Y., and N. M. are employees of Nippon Paint Co. Ltd. N. N., T. Y., W. N., Y. A., H. S., and A. O. declare no competing interests. The materials examined in this study were produced by Nippon Paint Co. Ltd. These factors do not alter adherence to Scientific Reports policies on sharing data and materials.
Figures



References
-
- Oie, S., Hosokawa, I. & Kamiya, A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J. Hosp. Infect.51, 140–143. 10.1053/jhin.2002.1221 (2002). - PubMed
-
- Weber, D. J., Anderson, D. & Rutala, W. A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis.26, 338–344. 10.1097/QCO.0b013e3283630f04 (2013). - PubMed
-
- Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: A common cause of persistent infections. Science284, 1318–1322. 10.1126/science.284.5418.1318 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

