Selective activation of SIGMAR1 in anterior cingulate cortex glutamatergic neurons facilitates comorbid pain in depression in male mice
- PMID: 39890921
- PMCID: PMC11785782
- DOI: 10.1038/s42003-025-07590-2
Selective activation of SIGMAR1 in anterior cingulate cortex glutamatergic neurons facilitates comorbid pain in depression in male mice
Abstract
Depression and comorbid pain are frequently encountered clinically, and the comorbidity complicates the overall medical management. However, the mechanism whereby depression triggers development of pain needs to be further elucidated. Here, by using the chronic restraint stress (CRS) mouse model of depression and comorbid pain, we showed that CRS hyperactivated the glutamatergic neurons in the anterior cingulate cortex (ACC), as well as increasing the dendrite complexity and number. Chemogenetic activation of these neurons can induce depression and pain, while chemogenetic blockade can reverse such depression-induced pain. Moreover, we utilized translating ribosome affinity purification (TRAP) in combination with c-Fos-tTA strategy and pharmacological approaches and identified SIGMAR1 as a potential therapeutic molecular target. These results revealed a previously unknown neural mechanism for depression and pain comorbidity and provided new mechanistic insights into the antidepressive and analgesic effects of the disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

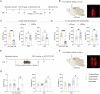
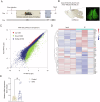
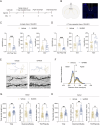
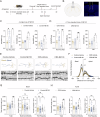
References
-
- Armbrecht, E. et al. Economic and humanistic burden associated with noncommunicable diseases among adults with depression and anxiety in the United States. J. Med. Econ.23, 1032–1042 (2020). - PubMed
-
- Von Knorring, L. et al. Pain as a symptom in depressive disorders. II. Relationship to personality traits as assessed by means of KSP. PAIN17, 377–384 (1983). - PubMed
-
- Gureje, O., Simon, G. E. & Von Korff, M. A cross-national study of the course of persistent pain in primary care. PAIN92, 195–200 (2001). - PubMed
-
- Carroll, L. J., Cassidy, D. J. & Côté, P. Depression as a risk factor for onset of an episode of troublesome neck and low back pain. PAIN107, 134–139 (2004). - PubMed
MeSH terms
Substances
Grants and funding
- 82101315/National Natural Science Foundation of China (National Science Foundation of China)
- 82204081/National Natural Science Foundation of China (National Science Foundation of China)
- 82130033/National Natural Science Foundation of China (National Science Foundation of China)
- 82293641/National Natural Science Foundation of China (National Science Foundation of China)
- 2022M722676/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Medical

