Endothelial and neuronal engagement by AAV-BR1 gene therapy alleviates neurological symptoms and lipid deposition in a mouse model of Niemann-Pick type C2
- PMID: 39891227
- PMCID: PMC11786545
- DOI: 10.1186/s12987-025-00621-4
Endothelial and neuronal engagement by AAV-BR1 gene therapy alleviates neurological symptoms and lipid deposition in a mouse model of Niemann-Pick type C2
Abstract
Background: Patients with the genetic disorder Niemann-Pick type C2 disease (NP-C2) suffer from lysosomal accumulation of cholesterol causing both systemic and severe neurological symptoms. In a murine NP-C2 model, otherwise successful intravenous Niemann-Pick C2 protein (NPC2) replacement therapy fails to alleviate progressive neurodegeneration as infused NPC2 cannot cross the blood-brain barrier (BBB). Genetic modification of brain endothelial cells (BECs) is thought to enable secretion of recombinant proteins thereby overcoming the restrictions of the BBB. We hypothesized that an adeno-associated virus (AAV-BR1) encoding the Npc2 gene could cure neurological symptoms in Npc2-/- mice through transduction of BECs, and possibly neurons via viral passage across the BBB.
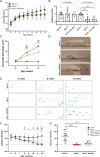
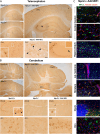
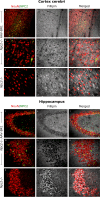
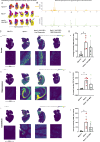
Methods: Six weeks old Npc2-/- mice were intravenously injected with the AAV-BR1-NPC2 vector. Composite phenotype scores and behavioral tests were assessed for the following 6 weeks and visually documented. Post-mortem analyses included gene expression analyses, verification of neurodegeneration in Purkinje cells, determination of NPC2 transduction in the CNS, assessment of gliosis, quantification of gangliosides, and co-detection of cholesterol with NPC2 in degenerating neurons.
Results: Treatment with the AAV-BR1-NPC2 vector improved motor functions, reduced neocortical inflammation, and preserved Purkinje cells in most of the mice, referred to as high responders. The vector exerted tropism for BECs and neurons resulting in a widespread NPC2 distribution in the brain with a concomitant reduction of cholesterol in adjacent neurons, presumably not transduced by the vector. Mass spectrometry imaging revealed distinct lipid alterations in the brains of Npc2-/- mice, with increased GM2 and GM3 ganglioside accumulation in the cerebellum and hippocampus. AAV-BR1-NPC2 treatment partially normalized these ganglioside distributions in high responders, including restoration of lipid profiles towards those of Npc2+/+ controls.
Conclusion: The data suggests cross-correcting gene therapy to the brain via delivery of NPC2 from BECs and neurons.
Keywords: AAV-BR1; Blood–brain barrier; Cholesterol; Cross-correction; Gangliosides; NPC2; Niemann-Pick type C2 disease; Viral gene therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal studies were performed according to the Danish Animal Experimentation Act (BEK no. 2028 of 14/12/2020) and the European directive (2010/63/EU) and carried out by licensed staff. The Danish Animal Experiments Inspectorate under the Ministry of Food, Agriculture and Fisheries has approved all animal experiments and breeding of NPC2-deficient mice (license no. 2018-15-0201-01467 and 2019-15-0202-00056). Consent for publication: Not applicable. Competing interests: JK is listed as an inventor on a patent on AAV-BR1, held by Boehringer Ingelheim International. All other authors have no competing interests to declare.
Figures





References
-
- Platt FM. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat Rev Drug Discov. 2018;17:133–50. - PubMed
-
- Verot L, Chikh K, Freydière E, Honoré R, Vanier MT, Millat G. Niemann-Pick C disease: functional characterization of three NPC2 mutations and clinical and molecular update on patients with NPC2. Clin Genet. 2007;71:320–30. - PubMed
-
- Berzina Z, Solanko LM, Mehadi AS, Jensen MLV, Lund FW, Modzel M, et al. Niemann-Pick C2 protein regulates sterol transport between plasma membrane and late endosomes in human fibroblasts. Chem Phys Lipids. 2018;213:48–61. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

