Glymphatic system clearance and Alzheimer's disease risk: a CSF proteome-wide study
- PMID: 39891246
- PMCID: PMC11786353
- DOI: 10.1186/s13195-024-01612-7
Glymphatic system clearance and Alzheimer's disease risk: a CSF proteome-wide study
Abstract
Background: The emerging evidence of the role of the glymphatic system (GS) in Alzheimer's disease (AD) provides new opportunities for intervention from the earliest stages of the disease. The aim of the study is to evaluate the efficacy of GS in AD to identify new disease biomarkers.
Methods: We performed a two-stage proteomic study to evaluate the GS health using intravenous gadolinium-based contrast agent (GBCA) with serial T1 3T magnetic resonance imaging (MRI) in individuals with amnestic mild cognitive impairment (aMCI). In Stage 1 (evaluated in the Cohort 1 of aMCI participants (n = 11)), we correlated the levels of 7K cerebrospinal fluid (CSF) proteins (estimated by SOMAscan) with GS health in 78 Freesurfer-segmented brain regions of interest (ROIs).
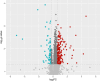
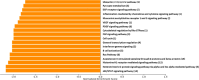
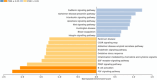
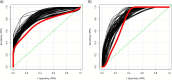
Results: A total of seven different proteins were significantly associated with GS health (p-value < 6.4 × 10-4). The stronger correlations were identified for NSUN6, GRAAK, OLFML3, ACTN2, RUXF, SHPS1 and TIM-4. A pathway enrichment analysis revealed that the proteins associated with GS health were mainly implicated in neurodegenerative processes, immunity and inflammation. In Stage 2, we validated these proteomic results in a new cohort of aMCI participants (with and without evidence of AD pathology in CSF (aMCI(-) and aMCI/AD( +); n = 22 and 7, respectively) and healthy controls (n = 10). Proteomic prediction models were generated in each ROI. These were compared with demographic-only models for identifying participants with aMCI(-) and aMCI/AD( +) vs controls. This analysis was repeated to determine if the models could identify those with aMCI/AD( +) from both aMCI(-) and controls. The proteomic models were found to outperform the demographic-only models.
Conclusions: Our study identifies proteins linked with GS health and involved the immune system in aMCI participants.
Keywords: Alzheimer’s disease; Glymphatic system; Inflammation; MRI; Mild cognitive impairment; Proteomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethical Committee of the Hospital Universitari MútuaTerrassa, Terrassa (Barcelona), Spain, and was conducted in accordance with both local regulations and international ethical standards, such as the Declaration of Helsinki. All participants were included after signing an informed consent form, having received comprehensive information regarding the study’s purpose, procedures, potential risks, and benefits. Confidentiality and anonymity of participants were strictly maintained throughout the study, and participants were informed of their right to withdraw at any point without consequence. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

