Intracellular Penetration of Atazanavir, Ritonavir and Dolutegravir With Concomitant Rifampicin: A Dose Escalation Study
- PMID: 39891354
- PMCID: PMC11993292
- DOI: 10.1002/cpt.3572
Intracellular Penetration of Atazanavir, Ritonavir and Dolutegravir With Concomitant Rifampicin: A Dose Escalation Study
Abstract
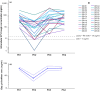
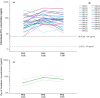
Ritonavir-boosted atazanavir is a victim of drug-drug interaction with rifampicin, a key component of antitubercular treatment. In a recent dose escalation clinical trial, we showed that increasing atazanavir/ritonavir to 300/100 mg b.i.d. compensates for reduced drug exposure in plasma due to rifampicin, but the intracellular effects remained unexplored. This sub-study investigated the intracellular penetration of atazanavir/ritonavir and dolutegravir into peripheral blood mononuclear cells (PBMC). Twenty-six healthy volunteers living with HIV, virologically suppressed, and taking atazanavir/ritonavir containing regimens were enrolled. The trial consisted of four sequential periods: PK1, participants were on atazanavir/ritonavir 300/100 mg q.d.; at PK2, rifampicin 600 mg q.d. and dolutegravir 50 mg b.i.d. were added (2 weeks); at PK3, atazanavir/ritonavir dose was increased to 300/100 mg b.i.d. (1 week); at PK4, rifampicin dose was doubled (1 week). Atazanavir, ritonavir, and dolutegravir were quantified in plasma and PBMC using LC-MS/MS methods to evaluate steady-state concentrations at the end of each period. Atazanavir/ritonavir dose escalation successfully restored intracellular concentrations comparable to those observed without rifampicin, with a geometric mean ratio of 0.99 (CI90 0.72-1.41) for atazanavir at PK3 compared with PK1. The intracellular concentration of dolutegravir increased significantly with atazanavir/ritonavir dose escalation, similar to plasma. Finally, further, increasing the rifampicin dose did not show an additional impact on atazanavir/ritonavir concentrations in PBMC. The study confirms that increasing the ATV/r dose can be an effective strategy for compensating rifampicin effects even at the intracellular level, supporting its use in clinical settings.
© 2025 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests in this work.
Figures
References
-
- WHO . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach (2021).
-
- WHO . Global Tuberculosis Programme—TB and HIV (2018).
-
- WHO . Global Tuberculosis Report (2020).
-
- Perloff, M.D. , Von Moltke, L.L. , Marchand, J.E. & Greenblatt, D.J. Ritonavir induces P‐glycoprotein expression, multidrug resistance‐associated protein (MRP1) expression, and drug transporter‐mediated activity in a human intestinal cell line. J. Pharm. Sci. 90, 1829–1837 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

