Identification of TTLL8, POTEE, and PKMYT1 as immunogenic cancer-associated antigens and potential immunotherapy targets in ovarian cancer
- PMID: 39891409
- PMCID: PMC11792853
- DOI: 10.1080/2162402X.2025.2460276
Identification of TTLL8, POTEE, and PKMYT1 as immunogenic cancer-associated antigens and potential immunotherapy targets in ovarian cancer
Abstract
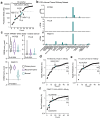
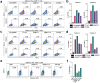
Most high-grade serous ovarian cancers (OC) do not respond to current immunotherapies. To identify potential new actionable tumor antigens in OC, we performed immunopeptidomics on a human OC cell line expressing the HLA-A02:01 haplotype, which is commonly expressed across many racial and ethnic groups. From this dataset, we identified TTLL8, POTEE, and PKMYT1 peptides as candidate tumor antigens with low expression in normal tissues and upregulated expression in OC. Using tissue microarrays, we assessed the protein expression of TTLL8 and POTEE and their association with patient outcomes in a large cohort of OC patients. TTLL8 was found to be expressed in 56.7% of OC and was associated with a worse overall prognosis. POTEE was expressed in 97.2% of OC patients and had no significant association with survival. In patient TILs, increases in cytokine production and tetramer-positive populations identified antigen-specific CD8 T cell responses, which were dependent on antigen presentation by HLA class I. Antigen-specific T cells triggered cancer cell killing of antigen-pulsed OC cells. These findings suggest that TTLL8, POTEE, and PKMYT1 are potential targets for the development of antigen-targeted immunotherapy in OC.
Keywords: Cancer immunology; immunotherapy; ovarian cancer; tumor antigen.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol: Off J Eur Soc Med Oncol. 2019;30(7):1080–1087. doi: 10.1093/annonc/mdz135. - DOI - PubMed
-
- Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, Yang P, Ruman J, Matei D. Pembrolizumab in patients with programmed death ligand 1–positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecologic Oncol. 2019;152(2):243–250. doi: 10.1016/j.ygyno.2018.11.017. - DOI - PubMed
-
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, Richardson GE, Sessa C, Yonemori K, Banerjee S, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi: 10.1016/S1470-2045(21)00216-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
