De novo variants in RYBP are associated with a severe neurodevelopmental disorder and congenital anomalies
- PMID: 39891528
- PMCID: PMC12228429
- DOI: 10.1016/j.gim.2025.101369
De novo variants in RYBP are associated with a severe neurodevelopmental disorder and congenital anomalies
Abstract
Purpose: Polycomb group proteins are key epigenetic transcriptional regulators. Multiple neurodevelopmental disorders are associated with pathogenic variants of the genes encoding Polycomb group proteins. RYBP is a core component of the noncanonical Polycomb Repressor Complex 1; however, its role in disease is unclear.
Methods: Functional consequences of RYBP variants were assessed using in vitro cellular and in vivo Drosophila melanogaster studies.
Results: We described 7 individuals with heterozygous de novo variants of RYBP and their clinical findings, including severe developmental delay, dysmorphisms, and multiple congenital anomalies. We showed that all single-nucleotide variants in RYBP localize to the N-terminal domain of the gene, which encodes the zinc-finger domain and ubiquitin-binding moiety. In vitro studies have demonstrated that the RYBP c.132C>G p.(Cys44Trp) variant causes reduced protein expression but does not affect the binding of YY1, RING1B, or ubiquitin. In vivo overexpression studies in Drosophila melanogaster showed a dramatic functional difference between human RYBP and its variant forms, affecting the C44 amino acid residue. DNA methylation studies suggested a possible episignature associated with RYBP-related disorder.
Conclusion: Heterozygous de novo variants in RYBP are associated with an identifiable syndromic neurodevelopmental disorder with multiple congenital anomalies.
Keywords: Epigenetic; Neurodevelopment; Polycomb; RYBP; Ubiquitin.
Copyright © 2025 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories.
Figures



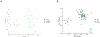


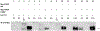
References
-
- Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017; 171(1): 34–57. - PubMed
-
- Busturia A, Morata G. Ectopic expression of homeotic genes caused by the elimination of the Polycomb gene in Drosophila imaginal epidermis. Development 1988; 104(4): 713–20. - PubMed
-
- Simoes da Silva CJ, Simon R, Busturia A. Epigenetic and non-epigenetic functions of the RYBP protein in development and disease. Mech Ageing Dev 2018; 174: 111–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

