Communicating a diagnosis of Dravet syndrome to parents/caregivers: An international Delphi consensus
- PMID: 39891606
- PMCID: PMC12014930
- DOI: 10.1002/epi4.13127
Communicating a diagnosis of Dravet syndrome to parents/caregivers: An international Delphi consensus
Abstract
Objective: Dravet syndrome is a developmental and epileptic encephalopathy characterized by drug-resistance, lifelong seizures, and significant comorbidities including intellectual and motor impairment. Receiving a diagnosis of Dravet syndrome is challenging for parents/caregivers, and little research has focused on how the diagnosis should be given. A Delphi consensus process was undertaken to determine key aspects for healthcare professionals (HCPs) to consider when communicating a Dravet syndrome diagnosis to parents/caregivers.
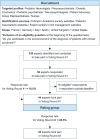
Methods: Following a literature search and steering committee review, 34 statements relating to the first diagnosis consultation were independent- and anonymously voted on (from 1, totally inappropriate, to 9, totally appropriate) by an international group of expert child neurologists, neuropsychiatrists, nurses, and patient advisory group (PAG) representatives. The statements were divided into five chapters: (i) communication during the first diagnosis consultation, (ii) information to be delivered during the first diagnosis consultation, (iii) points to be reiterated at the end of the first diagnosis consultation, (iv) information to be delivered at subsequent consultations, and (v) communication around genetic testing. Statements receiving ≥ 75% of the votes with a score of ≥7 and/or with a median score of ≥8 were considered consensual.
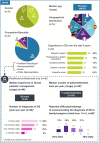
Results: The statements were evaluated by 44 HCPs and PAG representatives in the first round of voting; 29 statements obtained strong consensus, 3 received good consensus, and 2 did not reach consensus. The committee reformulated and resubmitted 4 statements for evaluation (42/44 voters): 3 obtained strong consensus and 1 remained not consensual. The final consensual recommendations include guidance on consultation setting, key disease aspects to convey, how to discuss genetic testing results, disease evolution, and the risk of SUDEP, among other topics.
Significance: It is hoped that this international Delphi consensus will facilitate a better-structured initial diagnosis consultation and offer further support for parents/caregivers at this challenging time of learning about Dravet syndrome.
Plain language summary: Diagnosis of Dravet syndrome, a rare and severe form of childhood-onset epilepsy, is often challenging to give to parents. This international study developed guidance and recommendations to help healthcare professionals better structure and personalize this disclosure. By following this advice, doctors can provide more tailored support to families, improving their understanding and management of the condition.
Keywords: Delphi consensus; Dravet syndrome; best practice recommendations; diagnosis communication; healthcare professional‐patient relationship.
© 2025 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Prof. AB has received honoraria for presenting at educational events, advisory boards and consultancy work for Biocodex, Encoded Therapeutics, Jazz/GW Pharma, Nutricia, Servier, Stoke Therapeutics, and UCB/Zogenix. Mrs. DB received honoraria for consulting and lecture from Eisai and Jazz Pharmaceuticals. Prof. FD received honoraria for consulting from Biocodex, UCB Pharma/Zogenix, Ethos srl, Neuraxpharm Italy S.p.A. Mrs. CE declares no conflict of interest. Mrs. SF declares no conflict of interest. Mr. AG declares no conflict of interest. Dr. KN received honoraria for consulting for Biocodex, UCB Pharma/Zogenix, Eyzs, Takeda, and Longboard. Prof. SSB received honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Biocodex, UCB Pharma, Desitin Arzneimittel, Eisai, Zogenix, GW/Jazz Pharmaceuticals, Ethypharm, Takeda, Marinus Pharma. Dr. ECM is a full‐time employee of Biocodex. Dr. RSC received honoraria for presentations and manuscript writing from Biocodex, UCB Pharma/Zogenix. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Sullivan J, Deighton AM, Vila MC, Szabo SM, Maru B, Gofshteyn JS, et al. The clinical, economic, and humanistic burden of Dravet syndrome ‐ a systematic literature review. Epilepsy Behav. 2022;130:108661. - PubMed
-
- Cooper MS, Mcintosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–47. - PubMed
-
- Makiello P, Feng T, Dunwoody B, Steckler F, Symonds J, Zuberi SM, et al. Comorbidities and predictors of health‐related quality of life in Dravet syndrome: a 10‐year, prospective follow‐up study. Epilepsia. 2023;64(4):1012–1020. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

