Impact of Headache and Over-the-Counter Treatment on Pain and Functional and Cognitive Parameters: A Real-World Study across Three Geographies
- PMID: 39891825
- PMCID: PMC11914668
- DOI: 10.1007/s40122-024-00703-5
Impact of Headache and Over-the-Counter Treatment on Pain and Functional and Cognitive Parameters: A Real-World Study across Three Geographies
Abstract
Introduction: Individuals with headache choose over-the-counter (OTC) medications to relieve pain and associated symptoms. This real-world evidence study investigated the effect of three OTC headache treatments on headache intensity and the associated impairment of cognitive and functional parameters in headache sufferers in Germany, Brazil, and Japan.
Methods: This prospective, multinational, observational eDiary-based study included adults experiencing headache for ≥ 6 months, with ≥ 2 headache episodes per month requiring treatment and using one of the three OTC headache treatments (Germany: ibuprofen 400 mg + caffeine 100 mg; Brazil: dipyrone 1 g; Japan: ibuprofen 100 mg + caffeine 40 mg). The primary endpoint was change in headache intensity (11-point numeric rating scale [NRS]) from baseline (headache onset) to 2 h post-treatment. Secondary endpoints were association between NRS scores for headache intensity and for cognitive and functional parameters and change in these parameters from baseline to 2 h post-treatment.
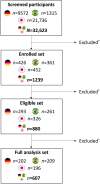
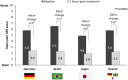
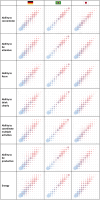
Results: Of the 32,623 individuals screened, 1239 were enrolled in the study, with 607 having their first headache episode treated using one of the OTC treatments. Baseline demographics and characteristics were similar across the cohorts. At 2 h post-treatment, headache intensity significantly improved, with the mean change from baseline being 3.4 (3.1, 3.7, 95% confidence interval), 4.2 (3.9, 4.5), and 3.0 (2.7, 3.3) for German, Brazilian, and Japanese cohorts, respectively. Improvement was observed in all cognitive and functional parameters. The NRS score for headache intensity significantly predicted NRS scores of all cognitive and functional parameters (P < 0.0001).
Conclusions: The study shows that headache intensity significantly affects cognitive and functional aspects, as well as overall quality of life, for sufferers globally. It confirms the effectiveness of OTC medications and suggests using headache intensity as a self-assessment tool for symptom severity, highlighting the need for new parameters in the OTC domain to improve public health benefits.
Keywords: Consumer healthcare; Headache; Migraine; Over-the-counter medication; Real-world evidence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Peter J. Goadsby reports, over the last 36 months, personal fees from Sanofi; grant from Kallyope, and personal fees from Aeon Biopharma, AbbVie, Aurene, CoolTech LLC, Dr Reddy’s, Eli Lilly and Company, Linpharma, Lundbeck, Pfizer, PureTech Health LLC, Satsuma, ShiraTronics, Teva Pharmaceuticals, Tremeau, and Vial; personal fees for advice from Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UptoDate, and Wolters Kluwer. Peter J. Goadsby is an Editorial Board member of Pain and Therapy. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Andrew Stewart has received honoraria for advisory boards and educational talks from Allergan/AbbVie, AllergoSan, Eli Lilly, Lundbeck, Novartis, Sanofi, and Teva. Mamoru Shibata has no competing interests. Mario Fernando Prieto Peres has received personal fees from Ache, Allergan/AbbVie, Eli Lilly, Eurofarma, Libbs, Lundbeck, Novartis, Pfizer, Sanofi, and Teva, and has received research support from Allergan/AbbVie, Eli Lilly, Eurofarma, Janssen-Cilag, Libbs, Natura, Novartis, and Teva. Mary Kay Margolis is a former employee of PPD, part of Thermo Fisher Scientific, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. As a PPD employee, she worked with a variety of companies and organizations and was expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. Chris Colby is an employee of PPD, part of Thermo Fisher Scientific. Valentine Polivka is an employee of the AIXIAL Group, an ALTEN company, which provides statistical consulting and research services to Sanofi, and has no other competing interests. Luminita Constantin, Caroline Amand, and Andrew Stewart are currently employees of Sanofi and may hold shares and/or stock options in the company. Ethical Approval: The study (21062-01) was submitted and approved by the US Institutional Review Board (IRB): Ethical and Independent (E&I) Review Services, which is now known as Salus IRB. The study was approved by local ethics committees in Germany, Brazil, and Japan. All participants provided informed consent electronically before initiation of data collection.
Figures


References
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. 10.1177/0333102417738202 - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. 10.1016/s0140-6736(18)32279-7. - PMC - PubMed
LinkOut - more resources
Full Text Sources

