Quantitative imaging outperforms No-reflow in predicting functional outcomes in a translational stroke model
- PMID: 39893086
- PMCID: PMC12014402
- DOI: 10.1016/j.neurot.2025.e00529
Quantitative imaging outperforms No-reflow in predicting functional outcomes in a translational stroke model
Abstract
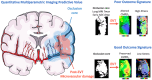
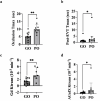
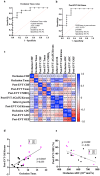
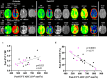
Microvascular dysfunction and no-reflow are considered a major cause of secondary damage despite revascularization in acute ischemic stroke (AIS), ultimately affecting patient outcomes. We used quantitative PET-MRI imaging to characterize early microvascular damages in a preclinical non-human primate model mimicking endovascular mechanical thrombectomy (EVT). During occlusion, PET perfusion and MRI diffusion were used to measure ischemic and lesion core volumes respectively. Following revascularization, multiparametric PET-MRI included perfusion, diffusion, blood-brain barrier (BBB) permeability MRI, and 15O-oxygen metabolism PET. Lesion growth on MRI was evaluated at one week, and the neurological score was assessed daily; a poor outcome was defined as a score>6 (0-normal, 60-death) after one week. Early after recanalization, the gold-standard PET ischemic threshold (<0.2 mL/min/g) identified post-EVT hypoperfusion in 67 % of the cases (14/21) located in the occlusion acute lesion. Acquired 110 min post-EVT, the area of MRI Tmax hypoperfusion was larger and even more frequent (18/20) and was also located within the acute lesion. Eight of the total cases (38 %) had a poor outcome, and all of them had no-reflow (7/8 MRI no-reflow and 6/8 PET no-reflow). Diffusion ADC alterations and post-EVT oxygen extraction fraction (OEF) values were significantly different in PET no-reflow cases compared to those without no-reflow, exhibiting an inverse correlation. Independently of no-reflow, long perfusion Tmax and post-EVT high BBB Ktrans in the lesion core were the hallmarks of poor outcome and infarct growth. This early quantitative imaging signature may predict infarct growth and poor outcome and help to identify neuroprotection targets.
Keywords: Acute ischemic stroke; Blood-brain barrier; No-reflow; Oxygen metabolism; PET-MRI; Thrombectomy.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Canet Soulas reports financial support was provided by French National Research Agency. Lucie Chalet reports a relationship with Olea Medical that includes: employment. Timothe Boutelier reports a relationship with Olea Medical that includes: employment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke [Internet] 2019 https://www.ahajournals.org/doi/10.1161/STR.0000000000000211 [cited 2022 Aug 31];50:344-418. - DOI
-
- Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. - PubMed
-
- Bai J., Lyden P.D. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10:143–152. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

