miR-142 deficit in T cells during blast crisis promotes chronic myeloid leukemia immune escape
- PMID: 39893171
- PMCID: PMC11787332
- DOI: 10.1038/s41467-025-56383-y
miR-142 deficit in T cells during blast crisis promotes chronic myeloid leukemia immune escape
Abstract
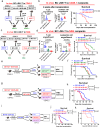
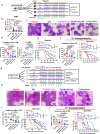
We reported that an acquired miR-142 deficit transforms chronic phase (CP) chronic myeloid leukemia (CML) leukemic stem cells (LSCs) into blast crisis (BC) LSCs. Given the role of miR-142 in the development and activity of the immune system, we postulated that this deficit also promotes LSC immune escape. Herein, we report on IL-6-driven miR-142 deficit occurring in T cells during BC transformation. In CML murine models, miR-142 deficit impairs thymic differentiation of lymphoid-primed multipotent progenitors (LMPP) into T cells and prevents T cells' metabolic reprogramming, thereby leading to loss of T cells and leukemia immune escape. Correcting miR-142 deficit with a miR-142 mimic compound (M-miR-142), alone or in combination with immune checkpoint antibodies, restores T cell number and immune activity, leading to LSC elimination and prolonged survival of BC CML murine and patient-derived xenograft models. These observations may open new therapeutic opportunities for BC CML and other myeloid malignancies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Rowley, J. D. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature243, 290–293 (1973). - PubMed
-
- Shrestha, A. et al. MicroRNA-142 is a multifaceted regulator in organogenesis, homeostasis, and disease. Dev. Dyn.246, 285–290 (2017). - PubMed
-
- Sharma, S. Immunomodulation: a definitive role of microRNA-142. Dev. Comp. Immunol.77, 150–156 (2017). - PubMed
-
- Kramer, N. J. et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood125, 3720–3730 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA286160/CA/NCI NIH HHS/United States
- R01 CA248475/CA/NCI NIH HHS/United States
- CA258981/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA258981/CA/NCI NIH HHS/United States
- CA248475/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

