MTOR Promotes Astrocyte Activation and Participates in Neuropathic Pain through an Upregulation of RIP3
- PMID: 39893345
- PMCID: PMC11787194
- DOI: 10.1007/s11064-025-04341-x
MTOR Promotes Astrocyte Activation and Participates in Neuropathic Pain through an Upregulation of RIP3
Abstract
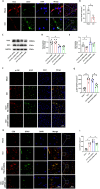
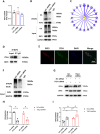
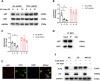
Neuropathic pain (NP), a chronic pain condition, is the result of abnormalities in both central and peripheral pain conduction pathways. Here, we investigated the underlying mechanisms associated with this effect. We found that following chronic constriction injury (CCI) surgery, there was an increase of mTOR in astrocytes and an activation of astrocytes within the spinal cord. Pharmacological inhibition of mTOR reversed CCI-induced hyperalgesia and neuroinflammation. Moreover, knockdown of astrocytic mTOR rescued the downregulation of spinal glutamate metabolism-related protein expression, underscoring the pivotal role of mTOR in modulating this pathway. Intriguingly, we observed that overexpression of mTOR, achieved via intrathecal administration of TSC2-shRNA, led to an upregulation of RIP3. Notably, pharmacological inhibition of RIP3, while ineffective in modulating mTOR activation, effectively eliminated the mTOR-induced astrocyte activation. Mechanistically, we found that mTOR controlled the expression of RIP3 in astrocytes through ITCH-mediated ubiquitination and an autophagy-dependent degradation. Taken together, our results reveal an unanticipated link between mTOR and RIP3 in promoting astrocyte activation, providing new avenues of investigation directed toward the management and treatment of NP.
Keywords: Astrocyte; MTOR; Neuroinflammation; Neuropathic pain; RIP3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests. Ethics Approval: All study protocols were reviewed and approved by Laboratory Animal Ethical and Welfare Committee of Shandong University Cheeloo College of Medicine (Approval No. 23056). Consent to Participate: Not applicable. Consent for Publication: Consent to publish was obtained from the participants.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

