Navigating a challenging path: precision disease treatment with tailored oral nano-armor-probiotics
- PMID: 39893419
- PMCID: PMC11786591
- DOI: 10.1186/s12951-025-03141-3
Navigating a challenging path: precision disease treatment with tailored oral nano-armor-probiotics
Abstract
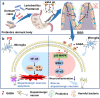
Oral probiotics have significant potential for preventing and treating many diseases. Yet, their efficacy is often hindered by challenges related to survival and colonization within the gastrointestinal tract. Nanoparticles emerge as a transformative solution, offering robust protection and enhancing the stability and bioavailability of these probiotics. This review explores the innovative application of nanoparticle-armored engineered probiotics for precise disease treatment, specifically addressing the physiological barriers associated with oral administration. A comprehensive evaluation of various nano-armor probiotics and encapsulation methods is provided, carefully analyzing their respective merits and limitations, alongside strategies to enhance probiotic survival and achieve targeted delivery and colonization within the gastrointestinal tract. Furthermore, the review explores the potential clinical applications of nano-armored probiotics in precision therapeutics, critically addressing safety and regulatory considerations, and proposing the innovative concept of 'probiotic intestinal colonization with nano armor' for brain-targeted therapies. Ultimately, this review aspires to guide the advancement of nano-armored probiotic therapies, driving progress in precision medicine and paving the way for groundbreaking treatment modalities.
Keywords: Gut to brain; Nanoparticles; Oral administration; Precision therapy; Probiotics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors gave their consent for publication. Competing interests: The authors declare no competing interests.
Figures






















Similar articles
-
Oral responsive delivery systems for probiotics targeting the intestinal tract.J Sci Food Agric. 2025 Aug 15;105(10):5133-5149. doi: 10.1002/jsfa.13938. Epub 2024 Oct 18. J Sci Food Agric. 2025. PMID: 39424610 Review.
-
Self-Nourishing and Armored Probiotics via Egg-Inspired Encapsulation.Adv Healthc Mater. 2025 Apr;14(11):e2405219. doi: 10.1002/adhm.202405219. Epub 2025 Mar 19. Adv Healthc Mater. 2025. PMID: 40103525
-
Single-cell encapsulation systems for probiotic delivery: Armor probiotics.Adv Colloid Interface Sci. 2024 Oct;332:103270. doi: 10.1016/j.cis.2024.103270. Epub 2024 Aug 6. Adv Colloid Interface Sci. 2024. PMID: 39142064 Review.
-
The Gut Microbiome Advances Precision Medicine and Diagnostics for Inflammatory Bowel Diseases.Int J Mol Sci. 2024 Oct 19;25(20):11259. doi: 10.3390/ijms252011259. Int J Mol Sci. 2024. PMID: 39457040 Free PMC article. Review.
-
Advances in CRISPR-Cas systems for gut microbiome.Prog Mol Biol Transl Sci. 2024;208:59-81. doi: 10.1016/bs.pmbts.2024.07.008. Epub 2024 Aug 22. Prog Mol Biol Transl Sci. 2024. PMID: 39266188 Review.
Cited by
-
Nanobiotechnology Unveils the Power of Probiotics: A Comprehensive Review on the Synergistic Role of Probiotics and Advanced Nanotechnology in Enhancing Geriatric Health.Cureus. 2025 Mar 12;17(3):e80478. doi: 10.7759/cureus.80478. eCollection 2025 Mar. Cureus. 2025. PMID: 40225478 Free PMC article. Review.
-
Modified-Release Pulmonary Delivery Systems for Labile Bioactives: Design, Development, and Applications.Pharmaceutics. 2025 Apr 3;17(4):470. doi: 10.3390/pharmaceutics17040470. Pharmaceutics. 2025. PMID: 40284465 Free PMC article. Review.
-
New advances in oral microbiology and tumor research.World J Clin Oncol. 2025 Jul 24;16(7):106981. doi: 10.5306/wjco.v16.i7.106981. World J Clin Oncol. 2025. PMID: 40741186 Free PMC article. Review.
References
-
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease [J]. Nat Rev Microbiol. 2021;19(1):55–71. - PubMed
Publication types
MeSH terms
Grants and funding
- 32401173/Starting Research Fund from the National Natural Science Foundation of China
- 30420220004/Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital
- 24GJHZ0072/Sichuan Science and Technology Program
- none/Sichuan Province Science and Technology Activities Funding for Returned Overseas Scholars
LinkOut - more resources
Full Text Sources

