Receipt of long-acting injectable antiretroviral therapy among people with HIV in Southern US states: an assessment using electronic health records and claims data
- PMID: 39893464
- PMCID: PMC11787751
- DOI: 10.1186/s12981-024-00690-9
Receipt of long-acting injectable antiretroviral therapy among people with HIV in Southern US states: an assessment using electronic health records and claims data
Abstract
Background: In January 2021, the United States (US) Food and Drug Administration (FDA) approved the first long-acting injectable antiretroviral therapy (LAI ART) regimen for the treatment of HIV providing an alternative to daily oral regimens. We analyzed electronic health records (EHRs) to provide real-world evidence of demographic and clinical characteristics associated with the receipt of LAI ART among people with HIV (PWH).
Methods: Leveraging EHRs from a large clinical research network in the Southern US - OneFlorida + linked with Medicaid (updated to 08/2022) - we identified a cohort of PWH who have been prescribed at least one dose of LAI ART since January 2021 and characterized their demographics, clinical characteristics, and HIV care outcomes.
Results: A total of 233 LAI ART recipients were identified: 56.7% female, 45.1% aged 30 to 44, 51.3% non-Hispanic Black, 78.1% on Medicaid and 4.7% on private insurance. Approximately three-quarters of injections (71.2%) were received within 37 days of the previous dose, and 84.4% were received within 67 days. About 8% of LAI ART recipients did not have optimal care engagement the year before LAI ART initiation; one in five recipients had a diagnosis of alcohol or substance use disorder in lifetime. All achieved viral suppression (< 50 copies/mL) before starting LAI ART. Of a subset of patients with HIV viral load test records, only 1 record of virologic failure (viral load > 200 copies/ml) was observed after the initiation of LAI ART.
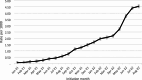
Discussion: There has been an increasing trend of LAI ART initiation since approval. People with suboptimal care engagement and with substance use disorder in lifetime were not excluded from LAI ART treatment.
Keywords: Cabotegravir/Rilpivirine; Electronic health records; HIV; Long-acting antiretroviral therapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors received research funding from Merck via its Investigator-Initiated Studies Program and received travel reimbursement from Merck to present related results at conferences.
Figures
References
-
- Centers for Disease Control and Prevention. Undetectable = Untransmittable. 2024 [cited 2024 Nov 20]; https://www.cdc.gov/global-hiv-tb/php/our-approach/undetectable-untransm...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical