Kaempferol enhances ER-mitochondria coupling and protects motor neurons from mitochondrial dysfunction and ER stress in C9ORF72-ALS
- PMID: 39893487
- PMCID: PMC11787762
- DOI: 10.1186/s40478-025-01927-y
Kaempferol enhances ER-mitochondria coupling and protects motor neurons from mitochondrial dysfunction and ER stress in C9ORF72-ALS
Abstract
Repeat expansions in the C9ORF72 gene are a frequent cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. Considerable progress has been made in identifying C9ORF72-mediated disease and resolving its underlying etiopathogenesis. The contributions of intrinsic mitochondrial deficits as well as chronic endoplasmic reticulum stress to the development of the C9ORF72-linked pathology are well established. Nevertheless, to date, no cure or effective therapy is available, and thus attempts to find a potential drug target, have received increasing attention. Here, we investigated the mode of action and therapeutic effect of a naturally occurring dietary flavanol, kaempferol in preclinical rodent and human models of C9ORF72-ALS. Notably, kaempferol treatment of C9ORF72-ALS human patient-derived motor neurons/neurons, resolved mitochondrial deficits, promoted resiliency against severe ER stress, and conferred neuroprotection. Treatment of symptomatic C9ORF72 mice with kaempferol, normalized mitochondrial calcium uptake, restored mitochondria function, and diminished ER stress. Importantly, in vivo, chronic kaempferol administration ameliorated pathological motor dysfunction and inhibited motor neuron degeneration, highlighting the translational potential of kaempferol. Lastly, in silico modelling identified a novel kaempferol target and mechanistically the neuroprotective mechanism of kaempferol is through the iP3R-VDAC1 pathway via the modulation of GRP75 expression. Thus, kaempferol holds great promise for treating neurodegenerative diseases where both mitochondrial and ER dysfunction are causally linked to the pathophysiology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Animal experiments: The study was approved by the Animal Commission of Canton of Bern, Switzerland, license number BE-35/17, BE-82/18. Human fibroblast-derived cells: Cells were anonymized and provided to us under an MTA from respective consortia. Culturing of cells only did not require internal review board (IRB) approval. Consent for publication: All authors have approved the manuscript and agree with its submission. Competing interests: The authors declare that they have no competing interests.
Figures
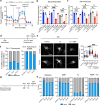

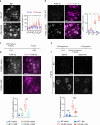
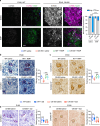
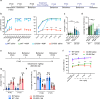

References
-
- Halliday M, Mallucci GR (2014) Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology 76:169–174 - PubMed
-
- Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13:477–491 - PubMed
-
- Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12:627–636 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

