Differential Expression/Regulation of Progesterone Receptor in Reproductive Tissues (Ovary and Uterus) Induced by Human Chorionic Gonadotropin and Equine Chorionic Gonadotropin Treatments in Sows
- PMID: 39893502
- PMCID: PMC11787484
- DOI: 10.1111/ahe.70017
Differential Expression/Regulation of Progesterone Receptor in Reproductive Tissues (Ovary and Uterus) Induced by Human Chorionic Gonadotropin and Equine Chorionic Gonadotropin Treatments in Sows
Abstract
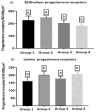
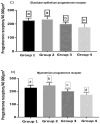
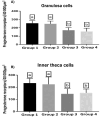
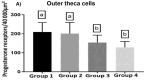
We studied sows (Landrace × Yorkshire line, DanBred Hybrid) to evaluate the possible changes in progesterone receptor (PR) expression in the uterus and ovary caused by different non-hypophyseal gonadotropins treatments: equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG). Varying concentrations of eCG and hCG were evaluated (Groups 1, 2, 3, 4). PR expression was determined by immunohistochemistry, and labelling intensity was determined by the HScore method. In the ovary, PR expression in the granulosa cells of follicles did not differ significantly between Groups 1 and 2 (p < 0.05) but differed significantly from that in Groups 3 and 4 (p < 0.05), which in turn did not differ from each other. This PR expression pattern was similar across groups in the internal and external theca cells. Conversely, in the uterus, PR expression in the lining epithelium was lower in Group 4 than that in Group 1 (p < 0.05). Increased expression was observed in the endometrial lamina propria in all groups 2 and 4 compared to that in the control group (p < 0.05). Decreased expression was observed in the glandular epithelium and myometrium in Group 4 compared to that in Group 1 (p < 0.05). In the ovary, PR expression in the granulosa and outer and inner theca of the follicles was not significantly different (p < 0.05) between Groups 1 and 2 or Groups 3 and 4; however, the expression in these pairs of groups differed from each other. Thus, changes in PR expression may depend on the concentrations and proportions of exogenous hormones used in the treatments, indicating an alteration in the reproductive process.
Keywords: hormones; ovary; progesterone; reproduction; sows; uterus.
© 2025 The Author(s). Anatomia, Histologia, Embryologia published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


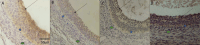
References
-
- Cuello, C. , Berthelot F., Martinat‐Botté F., et al. 2004. “Transfer of Vitrified Blastocysts From One or Two Superovulated Large White Hyperprolific Donors to Meishan Recipients: Reproductive Parameters at Day 30 of Pregnancy.” Theriogenology 61, no. 5: 843–850. 10.1016/S0093-691X(03)00257-7. - DOI - PubMed
-
- Esbenshade, K. L. , Ziecikt A. J., and Britt J. H.. 1990. “Regulation and Action of Gonadotrophinsin Pigs.” Journal of Reproduction and Fertility 40: 19–32. - PubMed
-
- Guthrie, H. D. , Pursel V. G., and Wall R. J.. 1997. “Porcine Follicle‐Stimulating Hormone Treatment of Gilts During an Altrenogest‐Synchronized Follicular Phase: Effects on Follicle Growth, Hormone Secretion, Ovulation, and Fertilization.” Journal of Animal Science 75, no. 12: 3246–3254. 10.2527/1997.75123246X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

