CCL21-CCR7 blockade prevents neuroinflammation and degeneration in Parkinson's disease models
- PMID: 39894839
- PMCID: PMC11789347
- DOI: 10.1186/s12974-024-03318-x
CCL21-CCR7 blockade prevents neuroinflammation and degeneration in Parkinson's disease models
Abstract
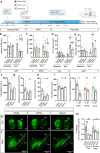
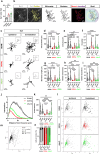
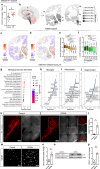
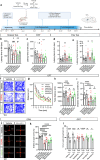
Parkinson's disease (PD) is a progressive degenerative disease of the central nervous system associated with neuroinflammation and microglial cell activation. Chemokine signaling regulates neuron-glia communication and triggers a microglial inflammatory profile. Herein, we identified the neuronal chemokine CCL21 as a major cause of microglial cell imbalance through the CCR7 receptor pathway with therapeutic implications for PD. In humans, we found that CCL21 transcript expression was increased in dopaminergic neurons (DANs) of the substantia nigra in PD patients. CCL21 and CCR7 expressions were spatially associated with brain regional vulnerability to synucleinopathies, as well as with the expression of microglial activation, neuroinflammation, and degeneration-related genes. Also, in mouse models of PD, we showed that CCL21 was overexpressed in DANs in vivo and in vitro. Mechanistically, neuronal CCL21 was shown to regulate microglial cell migration, proliferation, and activation in a CCR7-dependent manner through both canonical (PI3K/AKT) and non-canonical (ERK1/2/JNK) signaling pathways. Finally, we demonstrated that navarixin, a clinically relevant chemokine inhibitor with high affinity for the CCR7 receptor, could block CCL21 effects on microglia and prevent neurodegeneration and behavioral deficits in two mouse models of PD induced with either α-synuclein oligomers (αSynO) or 3,4-dihydroxyphenylacetaldehyde (DOPAL). Altogether, our data indicate that navarixin blocks CCL21/CCR7-mediated neuron-microglia communication and could be used as a therapeutic strategy against PD.
Keywords: CCL21-CCR7 pathway; Chemokine signaling; DOPAL; Microglia; Navarixin; Neuroinflammation; Parkinson`s disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All in vivo experiments were conducted following the Brazilian Experimental Animal Use Guidelines. This study was approved by the Ethics Committee of the Health Sciences Center of the Federal University of Rio de Janeiro (UFRJ) (Protocol number A6/19-001-16 and 025/15). Consent for publication: Not applicable. Clinical trial number: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the Elderly Research Group. Neurology. 2000;54:S21–3. - PubMed
-
- Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;2017 18:4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

