Direct Oral Anticoagulants Versus Vitamin K Antagonists After Mitral Valve Transcatheter Edge-to-Edge Repair in Patients With Atrial Fibrillation: A Single-Center Observational Study
- PMID: 39895535
- PMCID: PMC12074710
- DOI: 10.1161/JAHA.124.038834
Direct Oral Anticoagulants Versus Vitamin K Antagonists After Mitral Valve Transcatheter Edge-to-Edge Repair in Patients With Atrial Fibrillation: A Single-Center Observational Study
Abstract
Background: Mitral valve transcatheter edge-to-edge repair (M-TEER) has emerged as a viable therapy option in patients with severe mitral regurgitation and high surgical risk. Although atrial fibrillation is common among patients undergoing M-TEER, the optimal anticoagulatory treatment after the intervention is unknown.
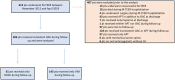
Methods: A single-center retrospective observational analysis was conducted using data from the M-TEER registry at the University Hospital Cologne collected from 2019 untill 2021 including patients undergoing M-TEER between November 2012 and April 2019. Patients with atrial fibrillation receiving consistent anticoagulation following M-TEER were categorized into a direct oral anticoagulant or a vitamin K antagonist (VKA) group. The primary end point was a composite of ischemic cerebrovascular and bleeding events. Additionally, overall survival was assessed.
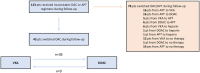
Results: Among 613 patients undergoing M-TEER, 206 met the inclusion criteria, with 61 receiving direct oral anticoagulants and 145 receiving VKAs. After a median follow-up of 833 (interquartile range, 355-1271) days, the incidence of the composite primary end point did not differ between direct oral anticoagulant and VKA groups (hazard ratio [HR], 0.51 [95% CI, 0.23-1.12]; P=0.07). Similarly, rates of ischemic cerebrovascular events and bleeding events were similar between groups. However, the overall mortality rate was higher in the VKA group (HR, 2.56 [95% CI, 1.54-4.26]; P=0.002). In the multivariable analysis, oral anticoagulation with a VKA was an independent predictor for death (adjusted HR, 2.23 [95% CI, 1.08-5.06]; P=0.03).
Conclusions: Our findings suggest that direct oral anticoagulants may offer comparable efficacy and safety to VKAs in preventing thromboembolic events following M-TEER in patients with atrial fibrillation. Further randomized trials are needed to confirm these results and establish optimal anticoagulation strategies in this patient population.
Keywords: bleeding events; direct oral anticoagulants; mitral regurgitation; mitral valve transcatheter edge‐to‐edge repair; thromboembolic events; vitamin K antagonists.
Conflict of interest statement
Dr Schipper received educational fees from Johnson & Johnson and Boston Scientific, and lecture fees from Abbott and Daiichi Sankyo. Dr Nies received travel support from Pfizer. Dr Wörmann received lecture fees from Abbott and Boston Scientific and educational fees from Boston Scientific and Johnson & Johnson. Dr Sultan received lecture fees from Medtronic, Boston Scientific, Abbott, and Johnson & Johnson. Dr Lüker received lecture fees from Johnson & Johnson, Abbott, and Boston Scientific. Dr Steven received lecture fees from Johnson & Johnson, Abbott, and Boston Scientific. Dr Hohmann received travel support, lecture honoraria, and personal fees from Edwards Lifesciences, Bayer, Daiichi Sankyo, Johnson & Johnson, MSD, and Pfizer. Dr Pfister received consultancy and speaker fees from Edwards Lifesciences, and speaker fees from Abbott. Dr Eitel received speaker honoraria from Abbott and Edwards Lifesciences. Dr Frerker received travel support and lecture honoraria from Abbott and Edwards Lifesciences. Dr Schmidt received travel support and lecture honoraria from Abbott and Edwards Lifesciences. The remaining authors have no disclosures to report.
Figures

References
-
- Goel SS, Bajaj N, Aggarwal B, Gupta S, Poddar KL, Ige M, Bdair H, Anabtawi A, Rahim S, Whitlow PL, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185–186. doi: 10.1016/j.jacc.2013.08.723 - DOI - PubMed
-
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000960 - DOI - PubMed
-
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

