Inotodiol Attenuates Mucosal Inflammation in a Mouse Model of Eosinophilic Chronic Rhinosinusitis
- PMID: 39895604
- PMCID: PMC11791372
- DOI: 10.4168/aair.2025.17.1.77
Inotodiol Attenuates Mucosal Inflammation in a Mouse Model of Eosinophilic Chronic Rhinosinusitis
Abstract
Purpose: Inotodiol (22-hydroxy lanosterol), a unique component of chaga mushrooms, is believed to be a medicinal component with reported antitumor, antiviral, and anti-inflammatory properties. This study evaluated the therapeutic potential and underlying mechanisms of inotodiol in eosinophilic chronic rhinosinusitis (ECRS).
Methods: An ECRS mouse model was established using female BALB/c mice. Forty mice were categorized into 4 groups: the control group (n = 10), ECRS group treated with solvent (n = 10), ECRS group treated with inotodiol 20 mg/kg (n = 10), and ECRS group treated with dexamethasone 10 mg/kg (n = 10). The nasal lavage fluid and tissue samples from mice were analyzed for cytokine and chemokine expression as well as for the severity of mucosal inflammation. Enzyme-linked immunosorbent assay, quantitative reverse transcription-polymerase chain reaction, histopathological staining, and immunofluorescence techniques were employed. The human eosinophil cell line (EoL-1) and dispersed nasal polyp cells (DNPCs) were used to assess inotodiol-induced eosinophil apoptosis in vitro via immunofluorescence, flow cytometry, and proteome profiler antibody array analysis.
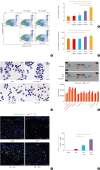
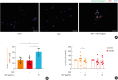
Results: Inotodiol significantly reduced the secretion of T2 cytokine and mast cell tryptase as well as the expression of Th cytokines, chemokines, and proinflammatory/inflammatory cytokines in ECRS mice. Furthermore, it suppressed mucosal inflammatory features such as polyp formation, epithelial thickening, and eosinophil infiltration. Inotodiol treatment reduced mast cell activation and increased eosinophil apoptosis in the nasal mucosa of ECRS mice. Notably, inotodiol also induced apoptosis in EoL-1 cells and DNPCs, which may contribute to its anti-inflammatory effects.
Conclusions: Inotodiol could be a potential therapeutic agent for ECRS by modulating immune responses and reducing mucosal inflammation.
Keywords: Apoptosis; cytokines; eosinophils; inflammation; inotodiol; mushroom; nasal polyp; rhinosinusitis.
Copyright © 2025 The Korean Academy of Asthma, Allergy and Clinical Immunology • The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures




References
-
- Ahn JC, Kim JW, Lee CH, Rhee CS. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean National Health and Nutrition Survey 2008–2012. JAMA Otolaryngol Head Neck Surg. 2016;142:162–167. - PubMed
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA²LEN study. Allergy. 2011;66:1216–1223. - PubMed
-
- Bachert C, Marple B, Schlosser RJ, Hopkins C, Schleimer RP, Lambrecht BN, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6:86. - PubMed
-
- Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. - PubMed

