Intracellular Biomacromolecule Delivery by Stimuli-Responsive Protein Vesicles Loaded by Hydrophobic Ion Pairing
- PMID: 39895718
- PMCID: PMC11780410
- DOI: 10.1021/acsomega.4c07666
Intracellular Biomacromolecule Delivery by Stimuli-Responsive Protein Vesicles Loaded by Hydrophobic Ion Pairing
Abstract
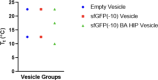
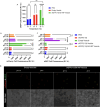
Proteins can perform ideal therapeutic functions. However, their large size and significant surface hydrophilicity and charge prohibit them from reaching intracellular targets. These chemical features also render them poorly encapsulated by nanoparticles used for intracellular delivery. In this work, a novel combination of protein vesicles and hydrophobic ion pairing (HIP) was used to load protein cargo and achieve cytosolic delivery to overcome the limitations of previous protein vesicle properties. Protein vesicles are thermally self-assembling nanoparticles made from elastin-like polypeptide (ELP) fused to an arginine-rich leucine zipper and a globular protein fused to a glutamate-rich leucine zipper. To impart stimuli-responsive disassembly, physiological stability, and small size, the ELP sequence was modified to include histidine and tyrosine residues. HIP was used to load and release protein cargo requiring endosomal escape for cytosolic function. HIP vesicles enabled delivery of cytochrome c, a cytosolically active protein, and a significant reduction in viability in both a traditional two-dimensional (2D) human cancer cell line culture and a biomimetic three-dimensional (3D) organoid model of acute myeloid leukemia. By examining the uptake of positively and negatively charged fluorescent protein cargos loaded by HIP, this work revealed the necessity of HIP for cytosolic cargo delivery and how HIP loading influences protein vesicle self-assembly and disassembly using microscopy, small-angle X-ray scattering, and nanoparticle tracking analysis. HIP protein vesicles have the potential to broaden the use of intracellular proteins as therapeutics for various diseases and extend protein vesicles to deliver other biomacromolecules, as the strategy developed here resulted in the first cytosolic protein cargo delivery using protein vesicles.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Self-Assembled Recombinant Elastin and Globular Protein Vesicles with Tunable Properties for Diverse Applications.Acc Chem Res. 2024 May 7;57(9):1227-1237. doi: 10.1021/acs.accounts.3c00694. Epub 2024 Apr 16. Acc Chem Res. 2024. PMID: 38624000 Free PMC article.
-
Rational design of elastin-like polypeptide fusion proteins to tune self-assembly and properties of protein vesicles.J Mater Chem B. 2023 Jul 12;11(27):6443-6452. doi: 10.1039/d3tb00200d. J Mater Chem B. 2023. PMID: 37357544
-
Protein Vesicles with pH-Responsive Disassembly.Biomacromolecules. 2022 Sep 12;23(9):3678-3687. doi: 10.1021/acs.biomac.2c00562. Epub 2022 Aug 9. Biomacromolecules. 2022. PMID: 35943848
-
Breaking free: endocytosis and endosomal escape of extracellular vesicles.Extracell Vesicles Circ Nucl Acids. 2023 Jun 30;4(2):283-305. doi: 10.20517/evcna.2023.26. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39697985 Free PMC article. Review.
-
Elastin-like polypeptide-based micelles as a promising platform in nanomedicine.J Control Release. 2023 Jan;353:713-726. doi: 10.1016/j.jconrel.2022.12.033. Epub 2022 Dec 15. J Control Release. 2023. PMID: 36526018 Review.
Cited by
-
A polymeric nanovesicle delivers sulfopin and gemcitabine to remodel tumor microenvironment for enhanced chemoimmunotherapy against orthotopic pancreatic cancer.Mater Today Bio. 2025 Jul 28;34:102153. doi: 10.1016/j.mtbio.2025.102153. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40799994 Free PMC article.
References
LinkOut - more resources
Full Text Sources
