Effective Components of Panax notoginseng- Salvia miltiorrhiza in the Treatment of Melasma and Its Experimental Study
- PMID: 39895736
- PMCID: PMC11780428
- DOI: 10.1021/acsomega.4c09799
Effective Components of Panax notoginseng- Salvia miltiorrhiza in the Treatment of Melasma and Its Experimental Study
Abstract
Purpose: The main purpose of this study was to predict and verify the active ingredients of Panax notoginseng-Salvia miltiorrhiza in melasma based on network pharmacology analysis and experimental verification.
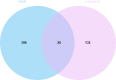
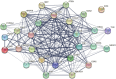
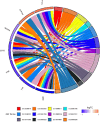
Materials and methods: Panax notoginseng-Salviae miltiorrhizae was investigated by network pharmacology, GEO database analysis, and molecular docking techniques to screen its active ingredients. The active components of Panax notoginseng-Salviae miltiorrhizae were further validated by an in vitro α-melanin-induced B16F10 melanoma cell model and an in vivo UV irradiation combined with a progesterone injection-induced melasma rat model.
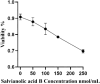
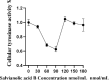
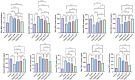
Results: Network pharmacology analysis and molecular docking showed that salvianolic acid B might be the key active ingredient. In vitro cellular experiments revealed that salvianolic acid B inhibits tyrosinase activity in B16F10 cells at concentrations of 60-90 nmol/mL. In vivo animal experiments found that TYR, MDA, and TNF-α were decreased in the skin and serum of rats in the group of the low-, medium-, and high-dose groups of salvianolic acid B, and the expression of GSH-Px and SOD was increased. The high-dose groups of salvianolic acid B showed the best therapeutic effect.
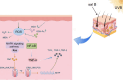
Conclusion: In this study, experiments collectively show that salvianolic acid B in Panax notoginseng-Salvia miltiorrhiza slows down the process of melasma by inhibiting lipid peroxidation in the organism, increasing the antioxidant capacity of the skin, decreasing the activity of tyrosinase, and providing anti-inflammation. This highlights the successful application of network pharmacology and provides a scientific basis for the clinical citation of Panax notoginseng-Salvia miltiorrhiza in treating melasma.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Shike S.; Yudi Z.; Rongheng L. Blood rheology and clinical. Modern Medical J. 2017, 45 (1), 163–167.
- Lin X. Y.; Zhou G. P.; Li L. A preliminary analysis of serum enzymes and hemorrheology for chloasma in female patients. J. Clin. Dermatol. 1997, (6), 359–361.
LinkOut - more resources
Full Text Sources
