Safety of dupilumab in Chinese pediatric patients aged 6 months and older: a prospective real-world study
- PMID: 39895989
- PMCID: PMC11782127
- DOI: 10.3389/fped.2024.1524962
Safety of dupilumab in Chinese pediatric patients aged 6 months and older: a prospective real-world study
Abstract
Objective: This study analyzes the occurrence and characteristics of adverse drug reactions (ADRs) of dupilumab in children in a real-world setting. It aims to enhance clinical practice and minimize medication safety risks in pediatric patients.
Methods: This prospective study included children receiving dupilumab in the hospital between January 2022 and December 2023. Information on ADRs was collected and univariate and multivariate analyses were employed to identify high-risk factors for the occurrence of adverse effects in dupilumab treatment.
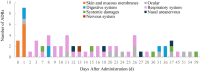
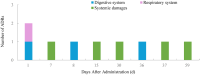
Results: A total of 65 ADRs occurred in 1,103 treatments in 127 patients, with an incidence of 27.56% (35/127). A total of 62 patients aged 6 or below participated in this study, accounting for 48.82%. Univariate analysis showed that gender, age, duration of medication, frequency of dupilumab use were risk factors for the occurrence of adverse effects (P < 0.05). Multivariate logistic regression analysis showed that age [odds ratio [OR]: 0.071, 95% confidence interval [CI]: 0.012-0.433; P = 0.004] and frequency of dupilumab use (OR: 3.306, 95% CI: 1.078-10.135; P = 0.036) were risk factors for adverse effects. The outcomes of ADRs were improved in 10 cases (15.38%) and completely recovered in 55 cases (84.62%).
Conclusion: Dupilumab has a good safety profile in Chinese children aged 6 months to 18 years for up to 2 years of treatment, with most adverse reactions being mild to moderate, and no serious ocular adverse reactions were reported. Age and frequency of dupilumab use were risk factors for adverse effects. Younger age and higher frequency of dupilumab use were associated with higher odds of ADRs.
Keywords: adverse drug reactions; children; dupilumab; real world; safety.
© 2025 Chen, Ni, Li, Hong, Zhu, Hong, Deng, Li, Pu, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources