Filamented hydrogels as tunable conduits for guiding neurite outgrowth
- PMID: 39896275
- PMCID: PMC11787030
- DOI: 10.1016/j.mtbio.2025.101471
Filamented hydrogels as tunable conduits for guiding neurite outgrowth
Abstract
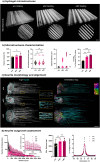
Anisotropic scaffolds with unidirectionally aligned fibers present an optimal solution for nerve tissue engineering and graft repair. This study investigates the application of filamented light (FLight) biofabrication to create hydrogel matrices featuring highly aligned microfilaments, facilitating neurite guidance and outgrowth from encapsulated chicken dorsal root ganglion (DRG) cells. FLight employs optical modulation instability (OMI) to rapidly and safely (<5 s) fabricate hydrogel constructs with precise microfilament alignment. The tunability of FLight matrices was demonstrated by adjusting four key parameters: stiffness, porosity, growth factor release, and incorporation of biological cues. Matrix stiffness was fine-tuned by varying the projection light dose, yielding matrices with stiffness ranging from 0.6 to 5.7 kPa. Optimal neurite outgrowth occurred at a stiffness of 0.6 kPa, achieving an outgrowth of 2.5 mm over 4 days. Matrix porosity was modified using diffraction gratings in the optical setup. While significant differences in neurite outgrowth and alignment were observed between bulk and FLight gels, further increases in porosity from 40 % to 70 % enhanced cell migration and axon bundling without significantly affecting maximal outgrowth. The incorporation of protein microcrystals containing nerve growth factor (NGF) into the photoresin enabled sustained neurite outgrowth without the need for additional NGF in the media. Finally, laminin was added to the resin to enhance the bioactivity of the biomaterial, resulting in a further increase in maximum neurite outgrowth to 3.5 mm after 4 days of culture in softer matrices. Overall, the varied matrix properties achieved through FLight significantly enhance neurite outgrowth, highlighting the importance of adaptable scaffold characteristics for guiding neurite development. This demonstrates the potential of FLight as a versatile platform for creating ideal matrices for clinical applications in nerve repair and tissue engineering.
Keywords: Dorsal root ganglion; Filamented light; Nerve growth factor; Neurite alignment.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Filamented Light (FLight) Biofabrication of Highly Aligned Tissue-Engineered Constructs.Adv Mater. 2022 Nov;34(45):e2204301. doi: 10.1002/adma.202204301. Epub 2022 Oct 9. Adv Mater. 2022. PMID: 36095325
-
Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation.Acta Biomater. 2019 Sep 15;96:175-187. doi: 10.1016/j.actbio.2019.06.035. Epub 2019 Jun 29. Acta Biomater. 2019. PMID: 31260823
-
Guidance of dorsal root ganglion neurites and Schwann cells by isolated Schwann cell topography on poly(dimethyl siloxane) conduits and films.J Neural Eng. 2011 Aug;8(4):046015. doi: 10.1088/1741-2560/8/4/046015. Epub 2011 Jun 15. J Neural Eng. 2011. PMID: 21673394
-
Effects of proinflammatory cytokines on axonal outgrowth from adult rat lumbar dorsal root ganglia using a novel three-dimensional culture system.Spine J. 2015 Aug 1;15(8):1823-31. doi: 10.1016/j.spinee.2015.03.017. Epub 2015 Mar 20. Spine J. 2015. PMID: 25797812
-
Matrix interactions modulate neurotrophin-mediated neurite outgrowth and pathfinding.Neural Regen Res. 2015 Apr;10(4):514-7. doi: 10.4103/1673-5374.155426. Neural Regen Res. 2015. PMID: 26170800 Free PMC article. Review.
Cited by
-
Structured Light Projection Using Image Guide Fibers for In Situ Photo-biofabrication.Adv Mater. 2025 Jul;37(27):e2419350. doi: 10.1002/adma.202419350. Epub 2025 Apr 29. Adv Mater. 2025. PMID: 40297914 Free PMC article.
References
-
- Pfister B.J., Gordon T., Loverde J.R., Kochar A.S., Mackinnon S.E., Kacy Cullen D. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011;39:81–124. doi: 10.1615/CritRevBiomedEng.v39.i2.20. - DOI - PubMed
LinkOut - more resources
Full Text Sources

