Fluvoxamine: First comprehensive insights into its molecular characteristics and inclusion complexation with β-cyclodextrin
- PMID: 39896317
- PMCID: PMC11787589
- DOI: 10.1016/j.jpha.2024.101040
Fluvoxamine: First comprehensive insights into its molecular characteristics and inclusion complexation with β-cyclodextrin
Abstract
Fluvoxamine (FXM) is a well-known selective serotonin reuptake inhibitor (SSRI) for treating depression and has recently been repurposed for efficacious treatment of coronavirus disease 2019. Although cyclodextrin (CD) encapsulation effectively improves the physicochemical properties of structurally diverse SSRIs, the molecular understanding of their associations is deficient. This comprehensive study used single-crystal X-ray diffraction integrated with density functional theory (DFT) calculation to provide deep insights into the conformationally flexible FXM and its inclusion complexation with β-CD. X-ray analysis revealed the first crystallographic evidence of the uncomplexed 3FXM-H+·3maleate- (1). Three FXM-H+ ions are counter-balanced by three planar maleate- ions to form a thin layer stabilized by infinite fused H-bond rings R4 4(12) and R6 4(16) and the interplay of π⋯π, CF⋯π and F⋯F interactions. For 2β-CD·2FXM-H+·maleate2-·23·2H2O (2), the tail-to-tail β-CD dimer encapsulates two FXM-H+ 4-(trifluoromethyl)phenyl moieties, which are charge-balanced by the rare non-planar maleate2- and stabilized by N/OH⋯O H-bonds and F⋯F interactions. This is a host-guest recognition pattern uniquely observed for all β-CD complexes with halogen (X)-bearing SSRIs, indicating the essence of X⋯X interactions and the shielding of X-containing moieties in the wall of the β-CD dimer. DFT calculations unveiled that the monomeric and dimeric β-CD-FXM complexes and FXM isomers are energetically stable, which alleviates the numbness and bitterness of the orally administered drug as previously patented. Additionally, an insightful conformational analysis of FXM emphasizes the importance of drug structural adaptation in pharmacological functions.
Keywords: Conformational flexibility; DFT calculation; Fluvoxamine maleate; SSRIs; X-ray analysis; β-Cyclodextrin.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
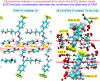

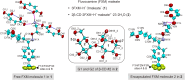
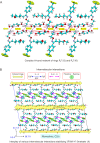




 N1 group are considered for the calculation of RMS fits. The corresponding RMS fit for each structure pair is indicated by nearby distance. Only site A of the twofold disordered CF3 group in 1 and 2 is shown.
N1 group are considered for the calculation of RMS fits. The corresponding RMS fit for each structure pair is indicated by nearby distance. Only site A of the twofold disordered CF3 group in 1 and 2 is shown.References
-
- World Health Organization (WHO) Depression and Other Common Mental Disorders: Global Health Estimates. 2017. http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng...
-
- WHO COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide. 2022. https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-i... - PMC - PubMed
-
- Brunton L., Knollman B., editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 14th ed. McGraw-Hill; 2023.
LinkOut - more resources
Full Text Sources

