This is a preprint.
Pancreatic injury induces β-cell regeneration in axolotl
- PMID: 39896453
- PMCID: PMC11785190
- DOI: 10.1101/2025.01.23.634564
Pancreatic injury induces β-cell regeneration in axolotl
Update in
-
Pancreatic injury induces β-cell regeneration in axolotl.Dev Dyn. 2025 Jul 18:10.1002/dvdy.70060. doi: 10.1002/dvdy.70060. Online ahead of print. Dev Dyn. 2025. PMID: 40679186 Free PMC article.
Abstract
Background: Diabetes is a condition characterized by a loss of pancreatic β-cell function which results in the dysregulation of insulin homeostasis. Using a partial pancreatectomy model in axolotl, we aimed to observe the pancreatic response to injury.
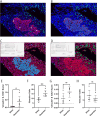
Results: Here we show a comprehensive histological assessment of pancreatic islets in axolotl. Following pancreatic injury, no apparent blastemal structure was observed. We found a significant, organ-wide increase in cellular proliferation post-resection in the pancreas compared to sham-operated controls. This proliferative response was most robust at the site of injury. We found that β-cells actively contributed to the increased rates of proliferation upon injury. β-cell proliferation manifested in increased β-cell mass in injured tissue at two weeks post injury. At four weeks post injury, we found organ-wide proliferation to be extinguished while proliferation at the injury site persisted, corresponding to pancreatic tissue recovery. Similarly, total β-cell mass was comparable to sham after four weeks.
Conclusions: Our findings suggest a non-blastema-mediated regeneration process takes place in the pancreas, by which pancreatic resection induces whole-organ β-cell proliferation without the formation of a blastemal structure. This process is analogous to other models of compensatory growth in axolotl, including liver regeneration.
Keywords: compensatory growth; diabetes; insulin; pancreatectomy; proliferation; resection.
Conflict of interest statement
Declaration of Interests JLW is a co-founder of Matice Biosciences. Other authors declare no competing interests.
Figures





References
-
- Vegas A.J., Veiseh O., Gürtler M., Millman J.R., Pagliuca F.W., Bader A.R., Doloff J.C., Li J., Chen M., Olejnik K., Tam H.H., Jhunjhunwala S., Langan E., Aresta-Dasilva S., Gandham S., Mcgarrigle J.J., Bochenek M.A., Hollister-Lock J., Oberholzer J., Greiner D.L., Weir G.C., Melton D.A., Langer R., Anderson D.G., 2016. Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nature Medicine 22, 306–311.. 10.1038/nm.4030 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
