This is a preprint.
Phosphatidylserine (PS)-targeting chimeric Interferon (IFN) fusion proteins for anti-tumor applications
- PMID: 39896467
- PMCID: PMC11785247
- DOI: 10.1101/2025.01.24.634764
Phosphatidylserine (PS)-targeting chimeric Interferon (IFN) fusion proteins for anti-tumor applications
Abstract
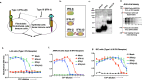
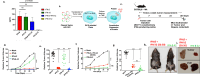
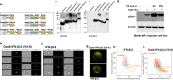
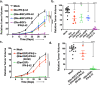
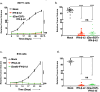
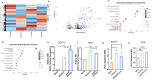
In viable healthy cells, membrane phospholipids are asymmetrically distributed across the lipid bilayer, whereby the anionic phospholipid phosphatidylserine is virtually all distributed on the inner leaflet of the plasma membrane. During apoptosis, phospholipid asymmetry collapses and PS is externalized to the external leaflet where it serves as an "eat-me" signal for efferocytosis, the process whereby dying cells are engulfed and degraded by phagocytes. PS is also externalized on viable activated tumor endothelial cells, stromal cells and cancer cells in the tumor microenvironment reflecting a pathophysiological state of solid cancers that function to suppress host anti-tumor immunity. Several strategies have been envisioned to target dysregulated PS in the tumor microenvironment including PS binding proteins such as Annexin V and PS-targeting monoclonal antibodies (Bavituximab) with promising preclinical results. Here, in an attempt to enhance the efficacy of PS-targeting therapeutics, we have generated a series of recombinant chimeric fusion proteins that fuse type I and type III IFNs (IFN-β-IFN-λ) into a single polypeptide chain separated by a short linker. The IFN-β-IFN-λ fusion proteins retain functions of both type I and type III IFNs but show combined effects to improve biological function as well as enhance anti-tumor activities. To localize IFNs to sites of externalized PS, we next fused the IFN-β-IFN-λ chimeric protein to the PS-targeting gamma-carboxyglutamic acid-rich (Gla) domain of Growth Arrest Specific factor 6 (Gas-6), rendering these IFN biologics as PS targeting modalities. Gas6-IFN-β-IFN-λ proteins selectively bind PS as evident by solid-phase ELISA assays as well as bind PS-positive cells, including apoptotic cells and cells that express CDC50 subunit mutant of the ATP11C flippase. In vivo, Gas6-IFN-β-IFN-λ retain strong anti-tumor activities in a syngeneic model when expressed ectopically in a E0771 breast cancer model and B16-F10 melanoma models. Collectively, we report on the generation and utility of a series of novel in class IFN fusion proteins that target the immune stimulatory features of IFNs to the PS externalization in the tumor microenvironment.
Keywords: Immune Escape; Immunogenic Biologicals; PS Targeting; PS receptors; Phosphatidylserine (PS); Type I Interferons; Type III Interferons.
Conflict of interest statement
Disclosure of potential conflicts of interest: SVK and RBB are cofounders of a biotechnology company called Targeron Therapuetics, LLC that aims to develop PS-targeting IFNs for immune-oncology applications,
Figures


References
-
- Agarwal D., et al. , Advances in Vaccines, Checkpoint Blockade, and Chimeric Antigen Receptor-Based Cancer Immunotherapeutics. Crit Rev Immunol, 2025. 45(1): p. 65–80. - PubMed
-
- Sharma P., et al. , Immune checkpoint therapy-current perspectives and future directions. Cell, 2023. 186(8): p. 1652–1669. - PubMed
-
- Sharma P., et al. , The Next Decade of Immune Checkpoint Therapy. Cancer Discov, 2021. 11(4): p. 838–857. - PubMed
-
- Budczies J., et al. , Tumour mutational burden: clinical utility, challenges and emerging improvements. Nat Rev Clin Oncol, 2024. 21(10): p. 725–742. - PubMed
-
- Fares C.M., et al. , Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am Soc Clin Oncol Educ Book, 2019. 39: p. 147–164. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
