This is a preprint.
Muscle Cathepsin B treatment improves behavioral and neurogenic deficits in a mouse model of Alzheimer's Disease
- PMID: 39896474
- PMCID: PMC11785056
- DOI: 10.1101/2025.01.20.633414
Muscle Cathepsin B treatment improves behavioral and neurogenic deficits in a mouse model of Alzheimer's Disease
Update in
-
Muscle Cathepsin B Treatment Improves Behavioral and Neurogenic Deficits in a Mouse Model of Alzheimer's Disease.Aging Cell. 2025 Nov;24(11):e70242. doi: 10.1111/acel.70242. Epub 2025 Oct 5. Aging Cell. 2025. PMID: 41047763 Free PMC article.
Abstract
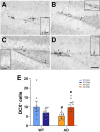
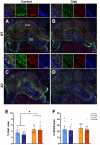
Muscle secretes factors during exercise that enhance cognition. Myokine Cathepsin B (Ctsb) is linked to memory function, but its role in neurodegenerative disease is unclear. Here we show that AAV-vector-mediated Ctsb overexpression in skeletal muscle in an Alzheimer's Disease (AD) mouse model (APP/PS1), improves motor coordination, memory function and adult hippocampal neurogenesis, while plaque pathology and neuroinflammation remain unchanged. Additionally, in AD mice, Ctsb treatment modifies hippocampal, muscle and plasma proteomic profiles to resemble that of wildtype controls. Conversely, in wildtype mice, Ctsb expression causes memory deficits and results in protein profiles across tissues that are comparable to AD control mice. In AD mice, Ctsb treatment increases the abundance of hippocampal proteins involved in mRNA metabolism and protein synthesis, including those relevant to adult hippocampal neurogenesis and memory function. Furthermore, Ctsb treatment enhances plasma metabolic and mitochondrial processes, and reduces inflammatory responses. In muscle, Ctsb expression elevates protein translation in AD mice, whereas in wildtype mice mitochondrial proteins decrease. Overall, the biological changes in the treatment groups are consistent with effects on memory function. Thus, skeletal muscle Ctsb application has potential as an AD therapeutic intervention.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
