This is a preprint.
Isolation and Bioassay of Linear Veraguamides from a Marine Cyanobacterium (Okeania sp.)
- PMID: 39896503
- PMCID: PMC11785078
- DOI: 10.1101/2025.01.18.633713
Isolation and Bioassay of Linear Veraguamides from a Marine Cyanobacterium (Okeania sp.)
Update in
-
Isolation and Bioassay of Linear Veraguamides from a Marine Cyanobacterium (Okeania sp.).Molecules. 2025 Feb 4;30(3):680. doi: 10.3390/molecules30030680. Molecules. 2025. PMID: 39942783 Free PMC article.
Abstract
Marine cyanobacteria have gained momentum in recent years as a source of novel bioactive small molecules. This paper describes the structure elucidation and pharmacological evaluation of two new, veraguamide O (1) and veraguamide P (2), and one known, veraguamide C(3), analogs isolated from a cyanobacterial collection made in the Las Perlas Archipelago of Panama. We hypothesized that these compounds would be cytotoxic in cancer cell lines. The compounds were screened against HEK-293, estrogen receptor positive (MCF-7), and triple-negative breast cancer (MDA-MB-231) cells as well as against a broad panel of membrane bound receptors. The planar structures were determined based on NMR and MS data along with comparison to previously isolated veraguamide analogs. Phylogenetic analysis of the collection suggests it to be an Okeania sp., a similar species to the cyanobacterium reported to produce other veraguamides. Veraguamide O shows no cytotoxicity (greater than 100 μM) against ER positive cells (MCF-7) with 13 μM IC50 against MDA-MB-231 TNBC cells. Interestingly, these compounds show affinity for the sigma2/TMEM-97 receptor making them potential leads for development of non-toxic sigma 2 targeting ligands.
Figures
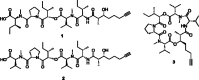
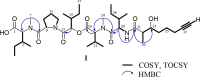

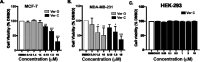

References
-
- Medina R. A.; Goeger D. E.; Hills P.; Mooberry S. L.; Huang N.; Romero L. I.; Ortega-Barria E.; Gerwick W. H.; McPhail K. L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J Am Chem Soc 2008, 130 (20), 6324–6325. DOI: 10.1021/ja801383f From NLM Medline. - DOI - PMC - PubMed
-
- Gutierrez M.; Tidgewell K.; Capson T. L.; Engene N.; Almanza A.; Schemies J.; Jung M.; Gerwick W. H. Malyngolide dimer, a bioactive symmetric cyclodepside from the panamanian marine cyanobacterium Lyngbya majuscula. J Nat Prod 2010, 73 (4), 709–711. DOI: 10.1021/np9005184 From NLM Medline. - DOI - PMC - PubMed
-
- Lax N. C.; Ahmed K. T.; Ignatz C. M.; Spadafora C.; Kolber B. J.; Tidgewell K. J. Marine cyanobacteria-derived serotonin receptor 2C active fraction induces psychoactive behavioral effects in mice. Pharm Biol 2016, 54 (11), 2723–2731. DOI: 10.1080/13880209.2016.1181659 From NLM Medline. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
