This is a preprint.
SLAMF6 enables efficient attachment, synapse formation, and killing of HIV-1-infected CD4+ T cells by virus-specific CD8+ T cells
- PMID: 39896504
- PMCID: PMC11785116
- DOI: 10.1101/2025.01.20.633914
SLAMF6 enables efficient attachment, synapse formation, and killing of HIV-1-infected CD4+ T cells by virus-specific CD8+ T cells
Abstract
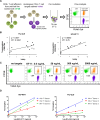
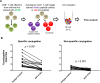
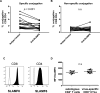
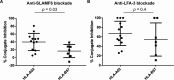
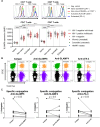
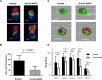
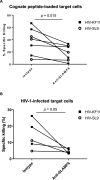
Efficient recognition and elimination of HIV-1-infected CD4+ T cells by cytotoxic CD8+ T cells (CTLs) require target cell engagement and the formation of a well-organized immunological synapse. Surface proteins belonging to the SLAM family are known to be crucial for stabilizing the immunological synapse and regulating antiviral responses during lymphotropic viral infections. In the context of HIV-1, there have been reports of SLAMF6 down-regulation in HIV-1-infected CD4+ T cells; however, the significance of this modulation for CTL function remains unclear. In this investigation, we used CTL lines from People living with HIV (PLWH) to examine the impact of SLAMF6 blockade on three pivotal processes: (1) the formation of CD8+-CD4+ T-cell conjugates, (2) the establishment of the immunological synapse, and (3) the killing and cytokine production capacity of HIV-1-specific CTLs during HIV-1 infection. Our findings reveal that the inability to form CD8+-CD4+ T-cell conjugates following incubation with an anti-SLAMF6 blocking antibody is primarily attributable to a defect in actin ring formation at the immunological synapse. Furthermore, SLAMF6 blockade leads to a reduction in the killing efficiency of HIV-1-infected CD4+ T cells by HIV-1-specific CTLs, underscoring the critical role of SLAMF6 in cytolytic function. This study highlights the importance of SLAMF6 receptors in modulating cytotoxic antiviral responses, shedding light on potential avenues for manipulation and enhancement of this pathway in the context of HIV and other lymphotropic viral infections.
Keywords: Cytokine production; Cytotoxic T lymphocytes (CTLs); Cytotoxic immune response; HIV-1; Immunological synapse; SLAMF6.
Figures
References
-
- Streeck H., Jolin J. S., Qi Y., Yassine-Diab B., Johnson R. C., Kwon D. S., Addo M. M., Brumme C., Routy J.-P., Little S., Jessen H. K., Kelleher A. D., Hecht F. M., Sekaly R.-P., Rosenberg E. S., Walker B. D., Carrington M., and Altfeld M.. 2009. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol 83: 7641–8. - PMC - PubMed
-
- Schmitz J. E., Kuroda M. J., Santra S., Sasseville V. G., Simon M. A., Lifton M. A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B. J., Ghrayeb J., Forman M. A., Montefiori D. C., Rieber E. P., Letvin N. L., and Reimann K. A.. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–60. - PubMed
-
- Schmitz J. E. 1999. Control of Viremia in Simian Immunodeficiency Virus Infection by CD8+ Lymphocytes. Science (1979) 283: 857–860. - PubMed
-
- Lim S. Y., Lee J., Osuna C. E., Vikhe P., Schalk D. R., Chen E., Fray E., Kumar M., Schultz-Darken N., Rakasz E., Capuano S., Ladd R. A., Gil H. M., Evans D. T., Jeng E. K., Seaman M., Martin M., Van Dorp C., Perelson A. S., Wong H. C., Siliciano J. D., Siliciano R., Safrit J. T., Nixon D. F., Soon-Shiong P., Nussenzweig M., and Whitney J. B.. 2024. Induction of durable remission by dual immunotherapy in SHIV-infected ART-suppressed macaques. Science 383: 1104–1111. - PMC - PubMed
-
- Seller Z. 2001. Cellular Adhesion and Adhesion Molecules. Turkish Journal of Biology 25: 1–15.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
