This is a preprint.
Chloride intracellular channel (CLIC) protein function in S1P-induced Rac1 activation requires membrane localization of the C-terminus, but not thiol-transferase nor ion channel activities
- PMID: 39896666
- PMCID: PMC11785189
- DOI: 10.1101/2025.01.22.634370
Chloride intracellular channel (CLIC) protein function in S1P-induced Rac1 activation requires membrane localization of the C-terminus, but not thiol-transferase nor ion channel activities
Update in
-
Chloride intracellular channel (CLIC) protein function in S1P-induced Rac1 activation requires membrane localization of the C-terminus, but not thiol-transferase nor ion channel activities.Front Cell Dev Biol. 2025 Apr 1;13:1565262. doi: 10.3389/fcell.2025.1565262. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40235733 Free PMC article.
Abstract
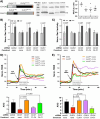
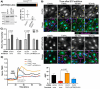
We have established a novel and evolutionarily-conserved function for chloride intracellular channel proteins (CLICs) in regulating Rho/Rac GTPases downstream of G protein-coupled receptors (GPCRs). Endothelial CLIC1 and CLIC4 are rapidly and transiently re-localized from the cytoplasm to the plasma membrane in response to the GPCR ligand sphingosine-1-phosphate (S1P), and both CLICs are required to activate Rac1 in response to S1P, but how they perform this function remains unknown. Biochemical studies suggest that CLICs act as non-specific ion channels and/or as glutathione-S-transferases, dependent on N-terminal features, in vitro. Here we investigate CLIC functional domains and membrane localization requirements for their function in S1P-mediated Rac1 signaling. Structure-function analyses of CLIC function in endothelial cells demonstrate that CLIC1 and CLIC4-specific functions reside at their C-termini, and that the CLIC4 N-terminus encodes determinants required for S1P-induced re-localization to the plasma membrane but is dispensable for S1P-induced Rac1 activation when the C-terminus is localized to the plasma membrane via a heterologous signal. Our results demonstrate that the postulated ion channel and thiol-transferase activities of CLICs are not required for Rac1 activation and suggests that sequences in the CLIC C-termini are critical for this function. Given the importance of S1P signaling in vascular biology and disease, our work establishes a platform to further our understanding of the membrane-localized proteins required to link GPCR activity to Rho/Rac regulation.
Keywords: CLIC1; CLIC4; Rac1; RhoA; S1P Receptor; endothelium; sphingosine-1-phosphate.
Conflict of interest statement
Conflict of Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
