Therapeutic plasma exchange accelerates immune cell recovery in severe COVID-19
- PMID: 39896810
- PMCID: PMC11782122
- DOI: 10.3389/fimmu.2024.1492672
Therapeutic plasma exchange accelerates immune cell recovery in severe COVID-19
Abstract
Background: Immunological disturbances (anti-type I IFN auto-antibody production, cytokine storm, lymphopenia, T-cell hyperactivation and exhaustion) are responsible for disease exacerbation during severe COVID-19 infections.
Methods: In this study, we set up a prospective, randomised clinical trial (ClinicalTrials.gov ID: NCT04751643) and performed therapeutic plasma exchange (TPE) in severe COVID-19 patients in order to decrease excess cytokines and auto-antibodies and to assess whether adding TPE to the standard treatment (ST, including corticosteroids plus high-flow rate oxygen) could help restore immune parameters and limit the progression of acute respiratory distress syndrome (ARDS).
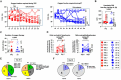
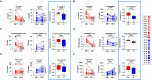
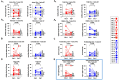
Results: As expected, performing TPE decreased the amount of anti-type I IFN auto-antibodies and improved the elimination or limited the production of certain inflammatory mediators (IL-18, IL-7, CCL2, CCL3, etc.) circulating in the blood of COVID-19 patients, compared to ST controls. Interestingly, while TPE did not influence changes in ARDS parameters throughout the protocol, it proved more effective than ST in reversing lymphopenia, preventing T-cell hyperactivation and reducing T-cell exhaustion, notably in a fraction of TPE patients who had an early favourable respiratory outcome. TPE also restored appropriate numbers of CD4+ and CD8+ T-cell memory populations and increased the number of circulating virus-specific T cells in these patients.
Conclusion: Our results therefore indicate that the addition of TPE sessions to the standard treatment accelerates immune cell recovery and contributes to the development of appropriate antiviral T-cell responses in some patients with severe COVID-19 disease.
Keywords: COVID-19; adaptive immunity; anti-type I IFN autoantibodies; cytokine storm; immune response; therapeutic plasma exchange.
Copyright © 2025 Guironnet-Paquet, Hamzeh-Cognasse, Berard, Cognasse, Richard, Yonis, Mezidi, Desebbe, Delannoy, Demeret, Marois, Saheb, Le, Schoeffler, Pugliesi, Debord, Bastard, Cobat, Casanova, Pescarmona, Viel, Nicolas, Nosbaum, Vocanson and Hequet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ, et al. . Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. (2021) 54:1257–75. doi: 10.1016/j.immuni.2021.05.010 - DOI - PMC - PubMed
-
- Zlei M, Sidorov IA, Joosten SA, Heemskerk MHM, Myeni SK, Pothast CR, et al. . Immune determinants of viral clearance in hospitalized COVID-19 patients: reduced circulating naïve CD4+ T cell counts correspond with delayed viral clearance. Cells. (2022) 11:2743. doi: 10.3390/cells11172743 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

