ADP101 multifood oral immunotherapy for food-allergic patients: Harmony phase 1/2 randomized clinical trial
- PMID: 39896962
- PMCID: PMC11786640
- DOI: 10.1016/j.jacig.2024.100382
ADP101 multifood oral immunotherapy for food-allergic patients: Harmony phase 1/2 randomized clinical trial
Abstract
Background: Oral immunotherapy is an established approach to desensitize the immune system in the context of allergic disease; however, the only currently approved product is for peanut allergy. ADP101 is a novel, pharmaceutical-grade, multifood oral immunotherapy in development to simultaneously treat single or multiple food allergies, containing allergenic proteins from 15 foods in equal parts by protein weight.
Objective: The phase 1/2 Harmony trial (NCT04856865) evaluated efficacy and safety of ADP101 in participants with qualifying allergy to 1 to 5 foods in ADP101, defined as dose-limiting symptoms with a ≤100 mg challenge dose during double-blind, placebo-controlled food challenge (DBPCFC).
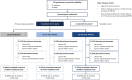
Methods: Participants were randomized to low-dose (1500 mg/d; 100 mg protein per food) or high-dose (4500 mg/d; 300 mg protein per food) ADP101, or matched placebo, with dose escalation followed by daily maintenance dosing over 40 weeks. The primary endpoint was the proportion of participants tolerating a ≥600 mg challenge dose of a single qualifying food without dose-limiting symptoms at the Week 40 Exit DBPCFC (ie, responders).
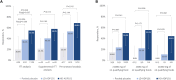
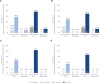
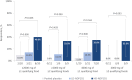
Results: In the primary analysis population (61 pediatric participants aged 4-17 years), a greater response rate was observed in both the high-dose ADP101 (55.0%) and low-dose ADP101 (38.1%) groups compared with pooled placebo (20.0%) (nominal P = .048, P = .306, respectively; adjusted for multiple comparisons, P = .097, P = .306, respectively). Desensitization to ≥2 foods was observed in individuals with multiple food allergies, as was desensitization at levels over 600 mg. ADP101-treated participants showed an overall reduction in skin-prick test reactivity, with an increase in maximum tolerated dose across the majority of foods tested. Adverse events were mostly mild or moderate, with no life-threatening events or deaths.
Conclusions: The study did not meet its primary endpoint, but ADP101 demonstrated a favorable safety profile and increased the reactive threshold in DBPCFC in pediatric participants with single or multiple food allergies across multiple endpoints, warranting further clinical investigation.
Keywords: Food allergy; allergy desensitization; clinical trial; multifood allergy; oral immunotherapy; pediatric food allergy.
© 2024 The Authors.
Conflict of interest statement
The Harmony trial was funded by Alladapt Immunotherapeutics Inc. Disclosure of potential conflict of interest: E. Kim is a consultant for ALK-Abello, Belhaven BioPharma, Cellergy Pharma, DBV Technologies, Genentech, Hanimune Therapeutics, Kenota Health, Novartis, 10.13039/501100001720Nutricia Research Foundation, Phylaxis, Revolo Biotherapeutics, and Ukko; and receives grant funding to his institution from the National Institute of Allergy and Infectious Diseases and 10.13039/100006423Food Allergy Research and Education (FARE). W. Carr is an employee of Allergy & Asthma Associates of Southern California; a researcher for Southern California Research; a consultant for Alladapt Immunotherapeutics Inc, Aluna, Hikma Pharmaceuticals, and Merck; an advisor for Amgen, AstraZeneca, and Hikma Pharmaceuticals; a speaker for AstraZeneca, Regeneron Pharmaceuticals, and Sanofi; and has an executive role/ownership interest in Allergy & Asthma Associates of Southern California. A. Assa’ad has received grant funding to their institution from AbbVie, Aimmune Therapeutics, Alladapt Immunotherapeutics Inc, DBV Technologies, FARE, 10.13039/100000002National Institutes of Health, Novartis, Sanofi, and Siolta; an internal research grant from Cincinnati Children’s Hospital Medical Centre; and is a holder of patent US7732135 (Genetic Marker of Food Allergy). S. Gogate has participated in advisory boards for IgGenix and Novartis. D. Petroni is a consultant for Aimmune Therapeutics, Alladapt Immunotherapeutics Inc, ARS Pharmaceuticals, DBV Technologies, Roche-Genentech, and Takeda Pharmaceuticals. T. Casale is a consultant for AstraZeneca, Genentech, Jasper Therapeutics, Lily, Novartis, Regeneron Pharmaceuticals, and Sanofi; and a member of the speakers’ bureau for Genentech and Novartis. O. Wang provided biostatistics support to Alladapt Immunotherapeutics Inc for the Harmony trial. M. Wang, A. Sullivan, A. Archer, C. Piscia-Nichols, L. Tuomi, O. Levin-Young, A. Dombkowski, and D. McClintock are current or previous employees of Alladapt Immunotherapeutics Inc.
Figures

References
LinkOut - more resources
Full Text Sources

