PPARGC1A regulates transcriptional control of mitochondrial biogenesis in early bovine embryos
- PMID: 39897080
- PMCID: PMC11782182
- DOI: 10.3389/fcell.2024.1531378
PPARGC1A regulates transcriptional control of mitochondrial biogenesis in early bovine embryos
Abstract
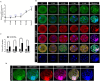
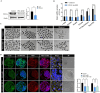
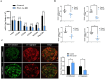
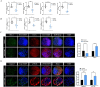
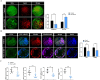
Extensive mitochondrial replication during oogenesis and its role in oocyte maturation and fertilization indicate that the amount of mitochondrial DNA (mtDNA) may play a significant role in early embryonic development. Early embryos express peroxisome proliferator-activated receptor gamma co-activator alpha (PPARGC1A/PGC-1a), a protein essential for mitochondrial biogenesis. This study investigated the role of PGC-1α from a single-cell zygotic stage to day-8 bovine blastocyst and the effect of PGC-1a knockdown (KD) on embryo mitochondria and development. PGC-1α KD via siRNA injection into single-cell zygotes does not substantially affect embryonic cleavage up to the morula stage but considerably reduces blastocyst development (18.42%) and hatching than the control (32.81%). PGC-1α regulates transcription of the gene encoding mitochondrial transcription factor A (TFAM), and immunofluorescence analysis indicated significantly lower TFAM expression in the 16-cell KD embryos and day-8 KD blastocysts. Reduction in NRF1 protein's nuclear localization in bovine blastomeres was also observed in PGC-1α-KD embryos. Furthermore, to understand the effect of PGC-1α-KD on the mitochondrial genome, we found a low mtDNA copy number in PGC-1α-KD day-8 bovine blastocysts. Several genes related to mitochondrial functioning, like ND1, ND3, ND5, ATPase8, COI, COII, and CYTB, were significantly downregulated in PGC-1α-KD embryos. Moreover, high mitochondrial depolarization (ΔΨm) and abnormal lipid depositions were observed in the PGC-1α KD blastocysts. SIRT1 is the upstream regulator of PGC-1α, but SIRT1 activation via Hesperetin does not affect PGC-1α-KD embryonic development considerably. In conclusion, PGC-1α plays a critical role in early embryo mitochondrial functioning, and any perturbation in its expression significantly disrupts early embryonic development.
Keywords: NRF; PGC-1α; TFAM; bovine blastocyst; mitochondrial DNA.
Copyright © 2025 Idrees, Haider, Perera, Ullah, Lee, Lee, Kang, Kim and Kong.
Conflict of interest statement
Author I-KK was employed by The King Kong Corp. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
LinkOut - more resources
Full Text Sources

