Benzimidazole resistance-associated mutations improve the in silico dimerization of hookworm tubulin: An additional resistance mechanism
- PMID: 39897360
- PMCID: PMC11784061
- DOI: 10.14202/vetworld.2024.2736-2746
Benzimidazole resistance-associated mutations improve the in silico dimerization of hookworm tubulin: An additional resistance mechanism
Abstract
Background and aim: Mutations in the β-tubulin genes of helminths confer benzimidazole (BZ) resistance by reducing the drug's binding efficiency to the expressed protein. However, the effects of these resistance-associated mutations on tubulin dimer formation in soil-transmitted helminths remain unknown. Therefore, this study aimed to investigate the impact of these mutations on the in silico dimerization of hookworm α- and β-tubulins using open-source bioinformatics tools.
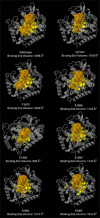
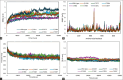
Materials and methods: Using AlphaFold 3, the α- and β-tubulin amino acid sequences of Ancylostoma ceylanicum were used to predict the structural fold of the hookworm tubulin heterodimer. The modeled complexes were subjected to several protein structure quality assurance checks. The binding free energies, overall binding affinity, dissociation constant, and interacting amino acids of the complex were determined. The dimer's structural flexibility and motion were simulated through molecular dynamics.
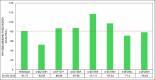
Results: BZ resistance-associated amino acid substitutions in the β-tubulin isotype 1 protein of hookworms altered tubulin dimerization. The E198K, E198V, and F200Y mutations conferred the strongest and most stable binding between the α and β subunits, surpassing that of the wild-type. In contrast, complexes with the Q134H and F200L mutations exhibited the opposite effect. Molecular dynamics simulations showed that wild-type and mutant tubulin dimers exhibited similar dynamic behavior, with slight deviations in those carrying the F200L and E198K mutations.
Conclusion: Resistance-associated mutations in hookworms impair BZ binding to β-tubulin and enhance tubulin dimer interactions, thereby increasing the parasite's ability to withstand treatment. Conversely, other mutations weaken these interactions, potentially compromising hookworm viability. These findings offer novel insights into helminth tubulin dimerization and provide a valuable foundation for developing anthelmintics targeting this crucial biological process.
Keywords: Ancylostoma; anthelmintic resistance; microtubules; soil-transmitted helminths.
Copyright: © Tenorio, et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- World Health Organization. Soil-transmitted Helminth Infections. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helmin... . Retrieved on 16-09-2023.
-
- Jourdan P.M, Lamberton P.H.L, Fenwick A, Addiss D.G. Soil-transmitted helminth infections. Lancet. 2018;391(10117):252–265. - PubMed
-
- Clements A.C.A, Alene K.A. Global distribution of human hookworm species and differences in their morbidity effects:A systematic review. Lancet Microbe. 2022;3(1):e72–e79. - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017:A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1798–1858. - PMC - PubMed
LinkOut - more resources
Full Text Sources
