Efficacy and retention rate of secukinumab in psoriatic arthritis across different clinical phenotypes: insights from the Italian GISEA Registry
- PMID: 39897378
- PMCID: PMC11783553
- DOI: 10.1177/1759720X251315138
Efficacy and retention rate of secukinumab in psoriatic arthritis across different clinical phenotypes: insights from the Italian GISEA Registry
Abstract
Background: Randomized clinical trials have demonstrated the efficacy of secukinumab (SECU) in reducing disease activity in psoriatic arthritis (PsA), while real-world studies prove a broader perspective on SECU's usefulness in everyday clinical practice.
Objectives: To assess the effectiveness of SECU by evaluating drug survival and identifying potential predictors of clinical response and treatment discontinuation in patients with moderate-to-severe PsA, using real-world data from the Italian Group for the Study of Early Arthritis (GISEA) registry.
Design: This longitudinal retrospective study included PsA patients treated with SECU, spanning from May 2016 to November 2023.
Methods: Data from 1045 PsA patients, including 783 with peripheral-only PsA (perPsA) and 262 with peripheral and axial involvement (mixed PsA) were analyzed. Drug survival was estimated by Kaplan-Meier analysis. Clinical outcomes, including Disease Activity Index for Psoriatic Arthritis (DAPSA), Psoriasis Area Severity Index (PASI), Ankylosing Spondylitis Disease Activity Score (ASDAS, C-Reactive Protein (CRP)-based), and Visual Analogue Scale (VAS) measures, were evaluated at baseline and at 6, 12, and 24 months. Adjusted hazard ratios (aHRs) for discontinuing SECU were determined using multivariate Cox regression models.
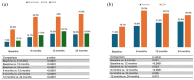
Results: SECU survival at 24 months was 63.24%, significantly higher in mixed PsA compared to perPsA (p = 0.036). In the overall PsA population, DAPSA scores decreased significantly at 6 months, and further at 24 months (all p < 0.0001). In mixed PsA, ASDAS-CRP scores were significantly reduced at 6 months and remained stable through 24 months (all p < 0.0001). VAS pain scores also improved already at 6 months and continued to improve at 24 months (all p < 0.0001). Higher age (aHR = 0.98, 95% confidence interval (CI): 0.96-0.99, p = 0.007) and lower baseline DAPSA scores (aHR = 1.02, 95% CI: 1.01-1.03, p = 0.014) were associated with greater persistence of SECU treatment. SECU was well tolerated, with no serious adverse events.
Conclusion: SECU showed sustained clinical improvements in both peripheral and axial involvement of PsA patients over 24 months, with higher persistence observed in mixed PsA patients. Our findings highlight the favorable clinical and safety profile of SECU in real world.
Keywords: axial disease; interleukin-17A; precision medicine; psoriatic arthritis; secukinumab.
© The Author(s), 2025.
Conflict of interest statement
F.I. and G.Lopalco received speaker honoraria from Novartis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Feld J, Ye JY, Chandran V, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford) 2020; 59(6): 1340–1346. - PubMed
-
- Mulder MLM, van Hal TW, van den Hoogen FHJ, et al. Measuring disease activity in psoriatic arthritis: PASDAS implementation in a tightly monitored cohort reveals residual disease burden. Rheumatology (Oxford) 2021; 60(7): 3165–3175. - PubMed
-
- Queiro R. Analysis of the HAQ-DI components in psoriatic arthritis patients with and without low disease impact. Rheumatology (Oxford) 2020; 59(11): 3569–3570. - PubMed
-
- Kerschbaumer A, Smolen JS, Ferreira RJO, et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2024; 83(6): 760–774. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

