Transplacental and genotoxicity effects of thallium(I) during organogenesis in mice
- PMID: 39897402
- PMCID: PMC11783430
- DOI: 10.1016/j.toxrep.2025.101896
Transplacental and genotoxicity effects of thallium(I) during organogenesis in mice
Abstract
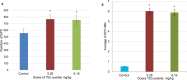
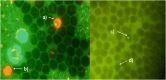
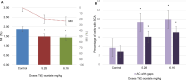
The increased concentration of thallium (Tl) in the environment is a cause for concern because the entire population, including pregnant women, is exposed, and this metal crosses the placenta and reaches the conceptus during development. In biological models such as mice, some abnormalities and delays in ossification occur in the fetuses of mice administered Tl on day 7 of gestation, but exposure to environmental Tl is constant during fetal development; therefore, in this study, the effects of several administrations of TI during organogenesis on the external morphology, skeletal development and genotoxicity of fetuses were evaluated. Four groups of 10 pregnant mice were administered 5.28, 6.16, 7.4 or 9.25 mg/kg body weight Tl(I) acetate intraperitoneally during fetal organogenesis. Additionally, samples were taken from fetuses from pregnant mice treated with 5.28 and 6.16 mg/kg body weight to evaluate the transplacental genotoxicity. The results revealed that the 9.25 mg/kg body weight dose produced maternal and fetal toxicity, and all of the treatment groups presented relatively high percentages of fetuses with external abnormalities, reduced bone ossification, and an increased percentage of liver cells with structural chromosomal aberrations (SCAs) and micronuclei (MNs) in blood cells. These results show that Tl(I) acetate administered during organogenesis produces abnormalities, including a delay in ossification and transplacental genotoxicity, in mouse fetuses. These findings are important because Tl has negative effects on development and may affect the health of offspring in the future because it can damage genetic material.
Keywords: Abnormalities in offspring; Chromosomal aberrations; Delayed ossification; Maternal toxicity; Micronucleus; Thallium(I) acetate.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Xia W., Du X., Zheng T., Zhang B., Bassig B.A., Zhou A., Wang Y., Xiong C., Li Z., Yao Y., Hu J., Zhou Y., Liu J., Xue W., Ma Y., Pan X., Peng Y., Xu S. A case-control study of prenatal thallium exposure and low birth weight in China. Environ. Health Perspect. 2016;124:164–169. doi: 10.1289/ehp.1409202. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

