Personalized allele-specific antisense oligonucleotides for GNAO1-neurodevelopmental disorder
- PMID: 39897576
- PMCID: PMC11787015
- DOI: 10.1016/j.omtn.2024.102432
Personalized allele-specific antisense oligonucleotides for GNAO1-neurodevelopmental disorder
Abstract
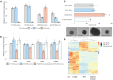
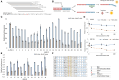
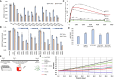
GNAO1-associated disorders are ultra-rare autosomal dominant conditions, which can manifest, depending on the exact pathogenic variant in GNAO1, as a spectrum of neurological phenotypes, including epileptic encephalopathy, developmental delay with movement disorders, or late-onset dystonia. There are currently no effective treatments available, apart from symptomatic options. In this work, we suggest harnessing personalized RNA therapy to treat GNAO1 patients and focus specifically on a recurrent pathogenic variant (E246K). We systemically screened allele-specific antisense oligonucleotides (ASOs) targeting the mutated allele to identify a potent and specific sequence using both reporter-based platforms and a patient-derived cellular model. We show that reduction of mutated GNAO1 in vitro by knockout or by ASO has a beneficial functional outcome, which can be measured by cAMP accumulation and gene expression changes. We established a Gnao1-E246K mouse model that shows a neurological phenotype, which partially recapitulates the human condition. Due to sequence similarity, the mouse can be treated with the selected ASO to test treatment efficacy in animal models, as shown in vitro using murine neural progenitor cells. Our results demonstrate a beneficial effect for the reduction of mutated GNAO1 by ASO in patient-derived models, demonstrating its feasibility as a therapeutic approach.
Keywords: ASOs; E246K; GNAO1; MT: Oligonucleotides: Therapies and Applications; allele-specific ASOs; antisense oligonucleotides; cellular models; individualized ASOs; mouse model; personalized ASOs.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

