miR-125b differentially impacts mineralization in dexamethasone and calcium-treated human mesenchymal stem cells
- PMID: 39897583
- PMCID: PMC11787018
- DOI: 10.1016/j.omtn.2024.102446
miR-125b differentially impacts mineralization in dexamethasone and calcium-treated human mesenchymal stem cells
Abstract
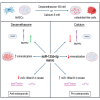
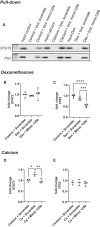
Bone metabolism is highly regulated, and microRNAs (miRs) can contribute to this process. Among them, miR-125b is well known to enhance osteoporosis and reduce osteogenic differentiation of human mesenchymal stem cells (hMSCs). In this work, we aim to evaluate and understand how miR-125b modulates mineralization of hMSCs in two different in vitro models. Cells were cultured in dexamethasone or calcium medium and transfected with miR-125b mimic. Exposure to dexamethasone or calcium medium increased the mineralization of hMSCs and was associated with decreased miR-125b expression. Transfection of miR-125b mimic in dexamethasone-treated cells increased mineralization, while it decreased it in calcium-treated cells. Levels of osteogenic markers presented the same difference. We identified STAT3, p53, and RUNX2 as direct targets of miR-125b in hMSCs. While these targets remained identical in both treatments, their modulation after transfection was different. We showed that miR-125b mimicking differentially modulated the expression of the miR-199a/214 cluster, probably via STAT3/miR-199a/214 and p53/miR-214 pathways. In conclusion, miR-125b affinity for targets implicated in bone remodeling changed depending on the in vitro models used to induce mineralization and led to opposite physiological effects. This work shows the complexity of drugs such as dexamethasone and opens the door for new in vitro models of mineralization.
Keywords: MT: Non-coding RNAs; calcium; dexamethasone; differential effect; miR-125b; mineralization; molecular mechanisms.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

