A patient-derived HCC spheroid system to model the tumor microenvironment and treatment response
- PMID: 39897611
- PMCID: PMC11782825
- DOI: 10.1016/j.jhepr.2024.101252
A patient-derived HCC spheroid system to model the tumor microenvironment and treatment response
Abstract
Background & aims: Hepatocellular carcinoma (HCC) is the third-leading and fastest rising cause of cancer-related death worldwide. The discovery and preclinical development of compounds targeting HCC are hampered by the absence of authentic tractable systems recapitulating the heterogeneity of HCC tumors in patients and the tumor microenvironment (TME).
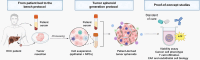
Methods: We established a novel and simple patient-derived multicellular tumor spheroid model based on clinical HCC tumor tissues, processed using enzymatic and mechanical dissociation. After quality controls, 22 HCC tissues and 17 HCC sera were selected for tumor spheroid generation and perturbation studies. Cells were grown in 3D in optimized medium in the presence of patient serum. Characterization of the tumor spheroid cell populations was performed by flow cytometry, immunohistochemistry (IHC), and functional assays. As a proof of concept, we treated patient-derived spheroids with FDA-approved anti-HCC compounds.
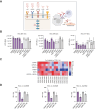
Results: The model was successfully established independently from cancer etiology and grade from 22 HCC tissues. The use of serum from patients with HCC was essential for tumor spheroid generation, TME function, and maintenance of cell viability. The tumor spheroids comprised the main cell compartments, including epithelial cancer cells, as well as all major cell populations of the TME [i.e. cancer-associated fibroblasts (CAFs), macrophages, T cells, and endothelial cells]. Tumor spheroids reflected HCC heterogeneity, including variability in cell type proportions and TME, and mimicked the original tumor features. Moreover, differential responses to FDA-approved anti-HCC drugs were observed between the donors, as observed in patients.
Conclusions: This patient HCC serum-tumor spheroid model provides novel opportunities for drug discovery and development as well as mechanism-of-action studies including compounds targeting the TME. This model will likely contribute to improve the therapeutic outcomes for patients with HCC.
Impact and implications: HCC is a leading and fast-rising cause of cancer-related death worldwide. Despite approval of novel therapies, the outcome of advanced HCC remains unsatisfactory. By developing a novel patient-derived tumor spheroid model recapitulating tumor heterogeneity and microenvironment, we provide new opportunities for HCC drug development and analysis of mechanism of action in authentic patient tissues. The application of the patient-derived tumor spheroids combined with other HCC models will likely contribute to drug development and to improve the outcome of patients with HCC.
Keywords: 3D model; Drug discovery and development; Immuno-oncology; Liver cancer; Tumor spheroids.
© 2024 Published by Elsevier B.V. on behalf of European Association for the Study of the Liver (EASL).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials

