Comparative evaluation of multimarker algorithms for early-stage HCC detection in multicenter prospective studies
- PMID: 39897614
- PMCID: PMC11782856
- DOI: 10.1016/j.jhepr.2024.101263
Comparative evaluation of multimarker algorithms for early-stage HCC detection in multicenter prospective studies
Abstract
Background & aims: We compared the clinical performance of the novel GAAD (gender [biological sex], age, alpha-fetoprotein [AFP], des-gamma carboxyprothrombin [DCP]) and GALAD (gender [biological sex], age, AFP, Lens culinaris agglutinin-reactive AFP [AFP-L3], DCP) algorithms to deduce the clinical utility of AFP-L3 for detecting early-stage hepatocellular carcinoma (HCC) from chronic liver disease (CLD).
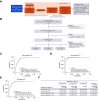
Methods: An algorithm development study (STOP-HCC-ARP) and clinical validation study (STOP-HCC-MCE) were conducted, recruiting adult participants with HCC (confirmed by radiology or pathology) or CLD in an international, multicenter, case-control design. Serum biomarkers were measured using Elecsys assays (GAAD and GALAD [Cobas]) or μTASWAKO assays (GALAD [μTASWAKO]) while blinded to case/control status.
Results: In STOP-HCC-ARP (algorithm development cohort), 1,006 patients {297 HCC (41.4% early-stage [Barcelona Clinic Liver Cancer {BCLC} 0/A) and 709 CLD} were included. Area under the curve (AUCs) for discriminating between early-stage HCC vs. CLD were 91.4%, 91.4%, and 90.8% for GAAD (Cobas), GALAD (Cobas), and GALAD (μTASWAKO), respectively. The clinical validation cohort of STOP-HCC-MCE comprised 1,142 patients, (366 HCC cases [48% early-stage], 468 specificity samples and 302 CLD); AUCs for GAAD (Cobas), GALAD (Cobas), and GALAD (μTASWAKO) for discriminating between early-stage HCC vs. CLD were 91.4%, 91.5%, and 91.0%, respectively; AUCs were 94.7-95.0% for all-stage HCC. The GAAD and GALAD algorithms demonstrated similar good performance regardless of disease etiology, presence of cirrhosis, geographical region, and within pan-tumor specificity panels (p <0.001).
Conclusions: GAAD (Cobas) demonstrated good clinical performance, similar to GALAD (Cobas and μTASWAKO) algorithms, in differentiating HCC and CLD controls, across all disease stages, etiologies, and regions; therefore, AFP-L3 may have a negligible role in GALAD for HCC surveillance.
Impact and implications: To improve the detection of early-stage hepatocellular carcinoma (HCC) from benign chronic liver disease (CLD), algorithms combining demographic characteristics and serum biomarkers, such as GAAD and GALAD, have been developed. GAAD combines gender (biological sex), age, alpha-fetoprotein (AFP), des-gamma carboxy-prothrombin (DCP); GALAD combines the same characteristics and biomarkers as GAAD with the addition of Lens culinaris agglutinin-reactive AFP (AFP-L3). Changing disease etiologies and treatment paradigms have raised questions regarding the utility of AFP-L3 in HCC surveillance. Our work demonstrates that the GAAD (Cobas) algorithm demonstrated good clinical performance and was as sensitive and specific as the GALAD (Cobas) and GALAD (μTASWAKO) algorithms in differentiating HCC and CLD controls, across all disease stages, etiologies, and geographical regions; therefore, AFP-L3 may have a negligible role in HCC detection. Our study provides supporting evidence that in participants with CLD undergoing guideline-directed HCC surveillance, the GAAD (Cobas) algorithm may be used as an effective method for the detection of HCC, potentially resulting in improved patient outcomes.
Keywords: Algorithm; GAAD; GALAD; Hepatocellular carcinoma; Surveillance.
© 2024 The Authors.
Conflict of interest statement
JH reports speaker’s bureau participation for Glaxo-Smith-Kline, Gilead Sciences, Roche Diagnostics, grant/research support from Gilead Sciences, BMS, advisory committee or review panel for Aligos, Assembly, Glaxo-Smith-Kline, Gilead Sciences, Johnson Pharmaceutica, and Roche. TB reports consultancy fees from Bayer, Eisai, Ipsen, Merck Sharp & Dome/Merck, Sirtex, and Roche. AV reports consultancy fees from AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, BMS, BTG, Daichi-Sankyo, EISAI, Incyte, Ipsen, MSD, PierreFabre, Roche, Servier, Sirtex, Tahio, Terumo. Speaker for AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, BMS, BTG, Daichi-Sankyo, EISAI, GSK, Imaging Equipment Ltd (AAA), Incyte, Ipsen, Jiangsu Hengrui Medicines MSD, PierreFabre, Roche, Servier, Sirtex, Tahio, Terumo. Research funding from Servier, and Incyte. Commercial medical education provider for Onclive, Oncowissen.de. TP reports speaker’s bureau participation for Bristol-Myers Squibb, Gilead Science, Bayer, Abbott, and Eisai, and MSD and research grant/contracts from Gilead Science, Roche Diagnostics, Jannsen Fibrogen, and VIR. JT reports consultancy fees for Amgen, Bayer Healthcare, Bristol-Myers Squibb, Eisai, Ipsen, Merck Serono, Merck Sharp & Dome, Lilly ImClone, and Roche. ENDeT has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, MSD, Mallinckrodt, Omega, Pfizer, Ipsen, Terumo and Roche and is currently employed by Boehringer-Ingelheim. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, AstraZeneca, BMS, Bayer, Celsion, and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, Ipsen, and Roche. MK reports speaking and teaching for Eisai, Bayer, Merck Sharp & Dome, Bristol-Myers Squibb, Eli Lilly & Co, and EA Pharma, and grant/research support from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, Eisai, Ono, and advisory committee or review panel for Eisai, Ono, MSD, Bristol-Myers Squibb, and Roche. KMal is an employee of Microcoat Biotechnologie, contracted by Roche Diagnostics. KMad, KK, and AS are employees of Roche Diagnostics International AG. PF, JKH, and WS have no conflicts to declare. HLYC reports consultancy fees from Arbutus Biopharma, Gilead Sciences, Glaxo-Smith-Kline, Roche, Vir Biotechnology, Aligos Therapeutics, Vaccitech, and Virion Therapeutics, and speaker’s bureau participation for Echosens, Gilead Sciences, Roche, and Viatris. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures






References
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
-
- Vogel A., Cervantes A., Chau I., et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):238–255. - PubMed
-
- European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

