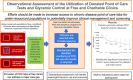
Observational assessment of the utilization of donated point of care tests and glycemic control at free and charitable clinics across the United States
- PMID: 39897629
- PMCID: PMC11782993
- DOI: 10.1016/j.plabm.2025.e00450
Observational assessment of the utilization of donated point of care tests and glycemic control at free and charitable clinics across the United States
Abstract
Introduction: Populations experiencing poverty often lack access to convenient lab tests. This analysis assesses trends observed from a national point of care (POC) lab donation program for free and charitable clinics across the United States.
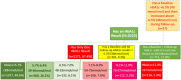
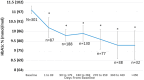
Methods: A total of 16 clinics were selected to receive a comprehensive package of POC lab tests. De-identified data on POC test utilization and results were assessed to descriptively identify trends in utilization (primary objective) and glycemic control (secondary objective). A paired t-test was utilized to identify statistically significant changes in HbA1c from baseline to predefined 90-day time intervals for all people living with diabetes (PLWD) and those with a baseline HbA1c ≥ 9.0 % (75 mmol/mol). The main comparison of interest was the change in HbA1c from baseline to 90-179 days.
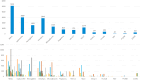
Results: A total of 17,563 POC tests were completed for 9658 patients with 3223 tests being HbA1c's. In the secondary analysis of PLWD with a baseline HbA1c ≥ 9.0 % (75 mmol/mol), patients who completed an HbA1c between 90 and 179 days (n = 188) demonstrated a statistically significant mean reduction from baseline of -1.2 % (95 % CI, -1.6 % to -0.9 %, p < 0.01, -10 mmol/mol [95 % CI, -6 mmol/mol - -14 mmol/mol]).
Discussion: The provision of POC labs helped support the care populations experiencing poverty received at free and charitable clinics, especially for chronic diseases like diabetes.
Keywords: Diabetes; Free and charitable clinics; HbA1c; Philanthropy; Point of care labs.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Sonak Pastakia reports financial support was provided by Heart to Heart International. Sonak Pastakia reports a relationship with Heart to Heart International that includes: consulting or advisory. SDP serves as a consultant for the philanthropic activities of other pharmaceutical companies including BD and Abbott. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Hall M.A. Rediscovering the importance of free and charitable clinics. N. Engl. J. Med. 2023;389(7):585–587. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

